Metal nanoparticles (NPs) are increasingly being used in many areas, e.g., industry, pharmacy, and biomedical engineering. NPs can be obtained through chemical and biological synthesis or using physical methods. AgNPs, AuNPs, CuNPs, FeNPs, MgNPs, SnO2NPs, TiO2NPs, and ZnONPs are the most commonly synthesized metal nanoparticles. Many of them have anti-microbial properties and documented activity supported by many tests against some species of pathogenic bacteria, viruses, and fungi. AgNPs, which are used for the production of commercial self-sterilizing packages, are one of the best-explored nanoparticles. Moreover, the EFSA has approved the use of small doses of silver nanoparticles (0.05 mg Ag·kg−1) to food products. Recent studies have shown that metal NPs can be used for the production of coatings to prevent the spread of the SARS-CoV-2 virus, which has caused the global pandemic. Some nanoparticles (e.g., ZnONPs and MgONPs) have the Generally Recognized As Safe (GRAS) status, i.e., they are considered safe for consumption and can be used for the production of edible coatings, protecting food against spoilage. Promising results have been obtained in research on the use of more than one type of nanometals, which prevents the development of pathogen resistance through various mechanisms of inactivation thereof.
1. Introduction
Microbiological stability is particularly important in the case of food with a high water content (e.g., fruit and vegetables). Various physical and chemical methods are used to ensure the microbiological purity of food. There is a trend towards minimization of the use of chemical preservatives and replacement thereof with natural substances. However, this does not always yield a fully satisfactory solution. The great interest in nanoparticles can be attributed to their physicochemical properties and wide application in many industries
[1][2][3][4][5]. In agricultural and production practice, cheap and easy protection measures against microorganisms are still a big challenge. New protection strategies against harmful microorganisms are continuously being developed. A promising approach may be the use of nanoparticles with antimicrobial and antiviral properties for the production of face masks, textiles, and other coatings
[6][7][8]. The use of metal nanoparticles for surface functionalization has been particularly successful in obtaining unique textiles with desirable properties, such as self-cleaning, antimicrobial, antifungal, flame retardant, ultraviolet blocking, and superhydrophobicity
[9]. The development of novel coatings that are safe, intelligent, and active is an important factor contributing to a reduction in food spoilage. The task of food coatings is to maintain the quality and properties of food products for as long as possible. Various additives are used to obtain intelligent and active food coatings. Nanoparticles occupy a special place among the huge variety of additives
[10][11]. The term “nanoparticles” refers to solid particles with a size between 1 and 100 nm
[12][13]. Nanoparticles usually have different properties than their macroscopic counterparts. In biological applications, the reduction in the size of metal particles to the nanometer scale is associated with an increase in their cytotoxicity. This is associated with their larger active surface and thus a higher reactivity than that of conventional materials and interactions with other compounds present in the environment. They also exhibit a higher bioavailability, which makes them easier to adsorb in specific organs, tissues, and cells
[14]. Another special active property of nanoparticles is their ability to absorb ultraviolet light, which is considered important for the prevention of photochemical reactions leading to the spoilage of food products. Nanoparticles also exert an antioxidant effect, which is a highly desirable property
[10]. Moreover, their most noticeable trait is the lower light scattering degree accompanying a reduction in the particle size. Sufficiently small particles can form transparent coatings. Nanoparticles do not directly induce transparency, but their light scattering effect declines with the decreasing particle size. At the same time, light scattering depends on the difference in the refractive index between the particle and the surrounding medium. A close match of refractive indices promotes the formation of transparent mixtures
[12]. Particles must be very well dispersed to achieve transparency in the nanoscale system, as agglomerated nanoparticles have the same optical properties as particles with the size of the agglomerate
[12].
Depending on their shape, nanoparticles are classified as zero-dimensional (0D), one-dimensional (1D), two-dimensional (2D), or three-dimensional (3D). This classification is associated with the movement of electrons in different planes. Electrons in 0D are trapped in a dimensionless space, whereas the electrons of 1D nanomaterials can move along the x-axis. 2D and 3D nanomaterials have electrons moving along the x-y axis and the x-y-z axis, respectively
[15]. One-dimensional nanomaterials form long nanostructures with thick membranes, i.e., nanotubes, nanofibers, nanowires, nanorods, and nanofilaments. Their length is greater than their width
[16]. In turn, 2D nanomaterials have sheet-like structures. These nanomaterials have the largest specific surface area of all known nanoparticles
[17]. Due to their high surface-to-volume ratio and the anisotropic physical and chemical properties, 2D nanomaterials are widely used against microorganisms and are attractive for food packaging applications. Large-area 2D nanomaterials directly interact with bacterial membranes, thereby enhancing the antibacterial effect
[18][19].
2. Biocompatibility of Metal Nanoparticles
Biocompatibility is the property of a material or substance functioning properly in a living organism. This means that, in addition to being present in living tissues, it must fulfill certain functions
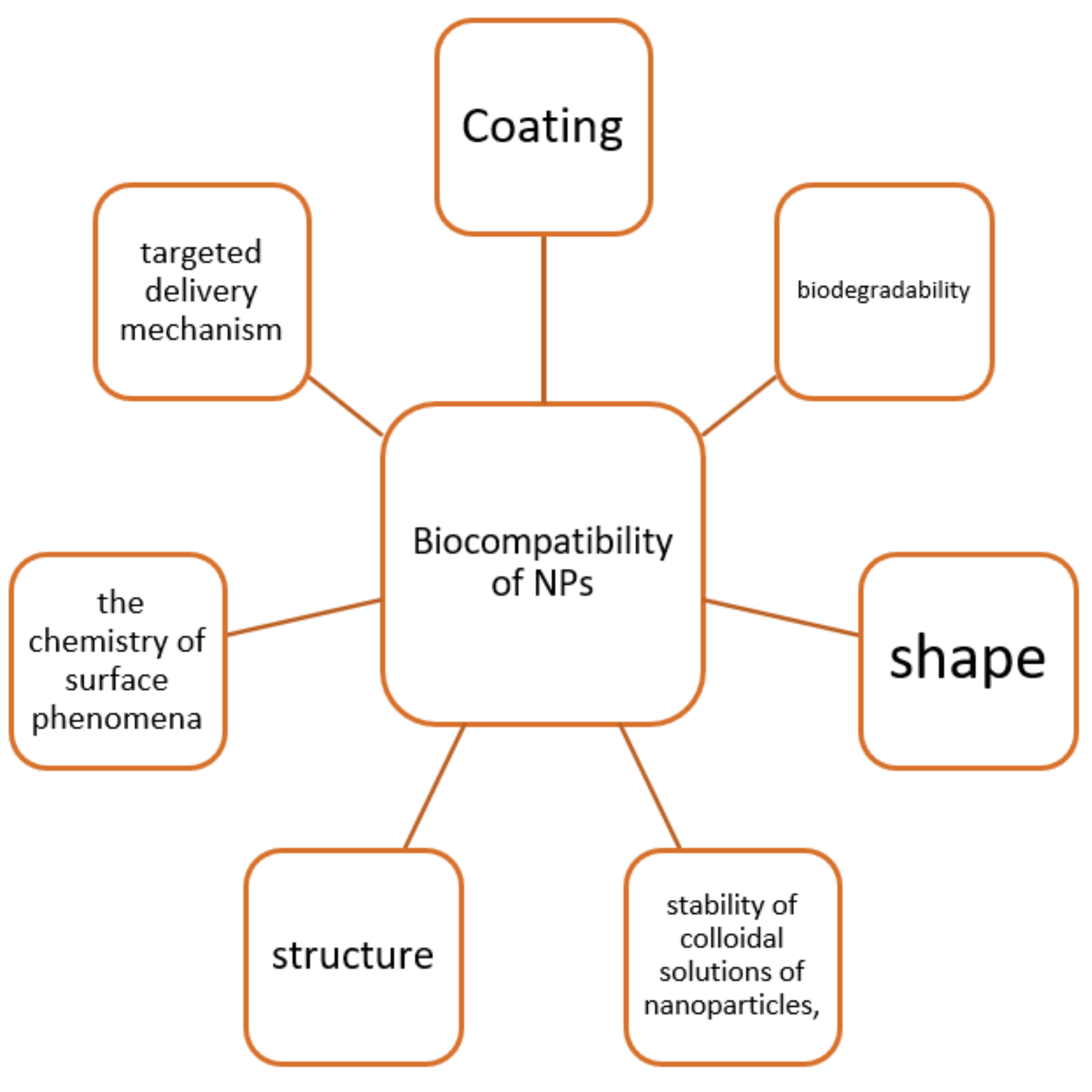
[20]. Biocompatibility depends on many factors: the targeted delivery mechanism, coating, biodegradability, chemistry of surface phenomena, structure, stability of nanoparticle colloidal solutions, and their ability to integrate into a cell or tissue without causing adverse effects (
Figure 1). A lack of biocompatibility for nanoparticles means a disruption of cellular and tissue metabolism leading to toxic effects in the body
[21].
Figure 1. Chosen properties determining the biocompatibility of nanoparticles.
Nanoparticles show a higher biocompatibility than metal ions, which can attack both normal mammalian cells and pathogenic bacteria. The biocompatibility of antimicrobial agents with metal ions is often a major clinical problem. Pure metal nanoparticles with the highest biocompatibility include AuNPS. Compared with Ag and Cu nanoparticles, Au nanoparticles are much more biocompatible and therefore represent a potentially more therapeutically promising antimicrobial agent. In addition to pure metals, nanoparticles also exist in the form of metal oxides. Among the most biocompatible ones is ZnONPs. It is characterized by a low toxicity to living cells and can be successfully used for drug delivery, edible coatings, and, supportively, as a cosmetic additive
[22]. Minimizing the toxicity of metal-based nanoparticles to normal eukaryotic cells without compromising their antimicrobial activity remains a major challenge. A future direction leading to an improved biocompatibility of nanoparticles may be the modification of their surface with polymers and peptides or polysaccharides
[23][24].
3. Use of Metal Nanoparticles in Protective Edible Coatings
The appropriate packaging and coating of fresh fruit and vegetables is essential to prevent quick drying, loss of firmness, and exposure to pathogens. Coatings have an impact on water vapor permeability, which should be low in order to reduce the drying rate. Additionally, the oxygen permeability should be reduced to slow down respiration without the creation of anaerobic conditions promoting the emergence of bad taste and production of ethanol. Ideal coatings ensure resistance and durability during the distribution and transport of food products and high antimicrobial properties to minimize microbial growth
[25]. Various active packaging systems are used to extend the shelf life of fruits and vegetables, e.g., oxygen and moisture absorbers, CO2 emitters, and antioxidant/antimicrobial composites. In recent years, an increase in the use of bio/polymer nanocomposites based on nanoparticles (NPs) has been reported. The use of NPs in bio/polymers offers many advantages over other packaging systems. Submicron-sized metal nanoparticles exhibit various possibilities for incorporation into food-grade polymer matrices serving as support systems. Metal nanosystems help to modify the properties of edible coatings. These properties are useful in solving food safety problems associated with pathogenic microorganisms, as they can limit the growth of yeasts, molds, and bacteria degrading the quality of food during storage and reducing the food shelf life
[10].
Table 1 presents some studies on bio-nanocomposite films and edible coatings used to improve physicochemical properties of food and extend its shelf life.
Table 1. Examples of the use of metal nanoparticles in edible coatings.
| NPs |
Coating |
Food |
References |
| ZnONps |
soy protein isolate |
banana |
[26] |
| chitosan/gum arabic |
[27] |
| chitosan |
papaya |
[28] |
| carboxymethyl-cellulose |
pomegranate fruit |
[29] |
| tomato |
[30] |
| chitosan/gum arabic |
avocado |
[31] |
| xanthan gum |
tomato and apple |
[32] |
| chitosan/alginate |
guava |
[33] |
| AgNps |
polylactic acid film |
mango |
[34] |
| polyvinylpyrrolidone |
asparagus |
[35] |
| carboxymethyl cellulose (CMC)-guar gum |
strawberry |
[36] |
| sodium alginate-gum |
black grapes |
[37] |
| TiO2NPs |
chitosan/polyvinyl alcohol |
cheese |
[38] |
| chitosan |
tomato |
[39] |
| Zn-MgONps |
alginate solution |
cold-smoked salmon |
[40] |
| AgTiO2NPs |
chitosan |
fruit |
[41] |
The use of metal nanoparticles in edible coatings poses several problems that have to be solved. One of them is associated with the achievement of uniform dispersion of NPs and the problem of the agglomeration of nanoparticles
[42]. Edible coatings must be appropriately applied to the raw material. The coating process consists of wetting the food surface with the coating solution followed by an adhesion step, in which, the coating solution penetrates the fruit or vegetable peel. Therefore, a high affinity between the coating solution and the food surface is necessary to facilitate the spread of the coating over the surface. Dipping, brushing, spraying, pan-coating, and fluidized the bed coating are well-known techniques, while electrostatic spraying has been used more recently
[43].
Another issue is the undesirable effect of metal nanoparticles on the human organism
[44]. Some nanoparticles have been approved for consumption, e.g., ZnONPs, which have been classified as GRAS by the FDA (21CFR73.1991) and as a safe additive (E6) by the EFSA
[45]. Silver is on the EFSA list of the Scientific Committee for Food (SCF), i.e., a group of additives with no established daily intake recommendations but with an accepted level limited to 0.05 mg Ag/kg food, based on a maximum concentration that does not cause any detectable adverse changes in morphology, functional capacity, growth performance, or survival in humans
[46].
Recently, due to the suspicion of some metal nanoparticles as carcinogens, many studies on non-toxicity and biocompatibility with the human body have been generated
[47]. Tan et al.
[48] investigated a polysaccharide coating based on metallic nanoparticles that was used as a packaging material to encapsulate curcumin. According to the authors, excellent biocompatibility properties were obtained. Pandey et al.
[49] tested a protein-based silver nanoparticle coating and found its low toxicity, effective application as a coating material for food products, and its high biocompatibility. Gnanasekar et al.
[50] developed a material containing palladium nanoparticles that was safe for use in food and did not interact with red blood cells. It also destroyed bacterial cells and showed anti-cancer properties. Li et al.
[51] used AuNPs to produce a β-glucan-based coating that was applied to edible mushrooms. This coating had beneficial effects in the gastrointestinal tract by causing the growth and activity of gut microbiota.
One of the trends in the latest research is the use of mixed nanomaterials, such as Zn-MgO, which is formed through association of zinc with highly biocompatible magnesium oxide (MgO) nanoparticles. The new material has been shown to retain strong antibacterial activity against Gram-positive bacteria, similar to ZnONPs, and to be safe for mammalian cells, similar to nano-MgO
[40].
When analyzing the suitability of metal nanoparticles for commercial application in the production of self-sterilizing surfaces, several issues must be considered. Modified surfaces or new biocidal formulations in general face some serious challenges before they reach the market. Problems such as manufacturing costs, production scalability, intellectual property issues, or regulatory aspects are just some of the major hurdles that these products will have to overcome in order to be commercialized
[52]. Some products, even with great lab results, fail when introduced on a larger scale due to high production costs, making them unattractive from a commercial standpoint. On the other hand, studies on the effects of a new product on human health are very time- and cost-intensive. However, the benefits of such materials may influence attempts to commercialize them
[53].
This entry is adapted from the peer-reviewed paper 10.3390/coatings12040480