Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Colorectal cancer (CRC) is a disorder that occurs exclusively in the colon or rectum and is caused by the colon’s aberrant proliferation of glandular epithelial cells.
- colon cancer
- oncology
- chemotherapy
- epidemiology
1. Introduction
Colorectal cancer (CRC), which comprises colon and/or rectum cancer, represents a significant health problem as the world’s third most commonly diagnosed and second most fatal cancer globally [1]. Approximately 9.4% of cancer-related deaths were due to CRC in 2020 [2]. However, in light of the significant increase in the number of identified cases in the older population, it is estimated that the global incidence of CRC will more than double by 2035, with the most significant increase occurring in less developed nations [3].
CRC is a disorder that occurs exclusively in the colon or rectum and is caused by the colon’s aberrant proliferation of glandular epithelial cells. There are three principal types of CRC: Sporadic, hereditary, and colitis-associated. The number of CRC cases is increasing globally day by day. Both environmental and genetic factors determine the risk of developing CRC. In addition, the risk of developing CRC in patients with long-standing ulcerative colitis and Crohn’s disease increases with age [4]. Multiple studies have demonstrated that risk factors for CRC include diet and lifestyle, family history, and chronic inflammation [5].
However, the most effective method of preventing CRC and reducing CRC-related deaths across the population is screening average-risk individuals [5]. Therefore, most European countries, Canada, specific regions in North and South America, Asia, and Oceania have initiated population-based screening programs [6]. Eligibility to participate in CRC screening is determined by age and area of residence. The results of microsimulation modeling have shown a downward trend in CRC morbidity and mortality in the United States, attributed to the implementation of screening programs [5]. In addition, population-based screening aims to reveal latent disease among the average-risk population, enabling early-stage interventions and reducing the threat to individuals and/or communities [7].
Screening is particularly appropriate in CRC, as it is not only a common disorder but one thought to be characterized by a gradual development of the adenoma–carcinoma sequence [8]. Although the time taken for an early adenoma to progress to an established CRC is yet unknown, the current evidence suggests it to be no less than ten years [9], offering abundant opportunity for detection via screening followed by treatment. Furthermore, CRC can be prevented by removing colorectal adenomas [10], and the earlier the CRC is detected, the less likely the patient will die [11]. Treatment outcomes are thus positively impacted by interventions along the adenoma–carcinoma pathway. Other effective strategies include identifying and monitoring high-risk populations, including individuals with inflammatory bowel disease, families with hereditary CRC syndrome, individuals whose family history suggests a genetic predisposition to CRC but have no detectable genetic markers, and individuals whose phenotypic appearance indicates high risk. The metabolome helped identify the important biological activities affected by genetic variation [12]. The most frequently used CRC screening methods are fecal occult blood tests (FOBTs) and lower endoscopy [13].
CRC development takes years. Generally, 10 to 15 years is required for a polyp to form a malignant tumor. So, regular screening, detecting, and removing polyps at the early stage is crucial; thereby, CRC can be prevented. Current diagnosis can detect only 40% of CRC cases in the early stages, and CRC might recur following surgery and post-surgery treatment [14]. Chemotherapeutic medications target cancer cells while also harming healthy cells in the environment. Modern chemotherapies acquired resistance in nearly all CRC patients, decreasing anticancer drug efficacy and ultimately leading to chemotherapy failure. Therefore, a comprehensive discussion on the epidemiology, the risk factors, and preventive measurement of CRC based on the updated evidence-based knowledge is crucial to overcome the future challenges of CRC. This research discussed the current global epidemiology, drug resistance, its challenges, risk factors, and preventive and treatment strategies of CRC. Additionally, there is a brief discussion on CRC carcinogenesis and recommendations for preventing and treating CRC.
2. Colorectal Cancer Development
CRC is genetically diverse; however, it can develop through various unique mechanisms. For example, many CRC cells exhibited dozens of somaclonal mutations resulting from the distinct level of gene expression profiles. As a result, CRC is believed to have one of the most incredible mutational loads of any malignancy. Based on the number of somaclonal mutations, CRC can be broadly categorized as hypermutated (more than 12 mutations per 106 bases) or non-hypermutated (fewer than 8.24 mutations per 106 bases) [15]. In addition, a novel categorization system for CRC was developed as a result of parallel efforts to categorize CRC based on gene expression profiles. These classifications have been updated and modified due to integrating data on gene expression profiles with tumor genotypes [15].
CRC develops when epithelial cells acquire a series of genetic or epigenetic changes that enable them to be hyperproliferative [16]. These rapidly developing cells form a benign adenoma, which can advance to cancer and metastasize via several distinct pathways, including microsatellite instability (MSI), chromosomal instability (CIN), and serrated neoplasia [17][18][19]. Adenoma–carcinoma sequence is a term used to describe cancer progression. The traditional pathway is responsible for the vast majority of sporadic CRC cases. Cancer begins as a tiny adenoma that becomes a giant adenoma and, finally, cancer. There is a strong association between this pathway and the development of the chromosomal instability (CIN)-positive subtype (CIN-positive). According to the National Cancer Institute, this model accounts for 10–15% of sporadic CRC. It is defined by development from normal cells to hyperplastic polyp, sessile serrated adenomas, and eventually cancer [13]. This route is typically involved in developing the CpG island methylator phenotype (CIMP)-high subtype, the pathway involved in inflammation. The prolonged inflammation causes normal cells to develop indeterminate dysplasia, which progresses further to low-grade dysplasia, progresses further to high-grade dysplasia, and finally, cancer [13]. Inflammatory bowel illnesses and the widespread use of prophylactic colectomy are responsible for less than 2% of CRC cases worldwide. There are benign precursor lesions accessible and removed in all pathways, albeit more noticeable in the adenoma–carcinoma and serrated pathways [13]. Because they take years to grow into cancer, there is a window of opportunity for secondary prevention of colorectal cancer.
When an adenocarcinoma becomes invasive, it can spread to other body parts via blood and lymphatic arteries (Figure 1). Adenocarcinomas account for roughly 96% of all CRC [20]. However, up to 18 years may pass between developing a polyp and invasive cancer. On average, it takes nine years to form metastasis [21]. Like any other tumor or cancer, CRC is classified by stage 0 (carcinoma in situ) through stage IV (Figure 1).
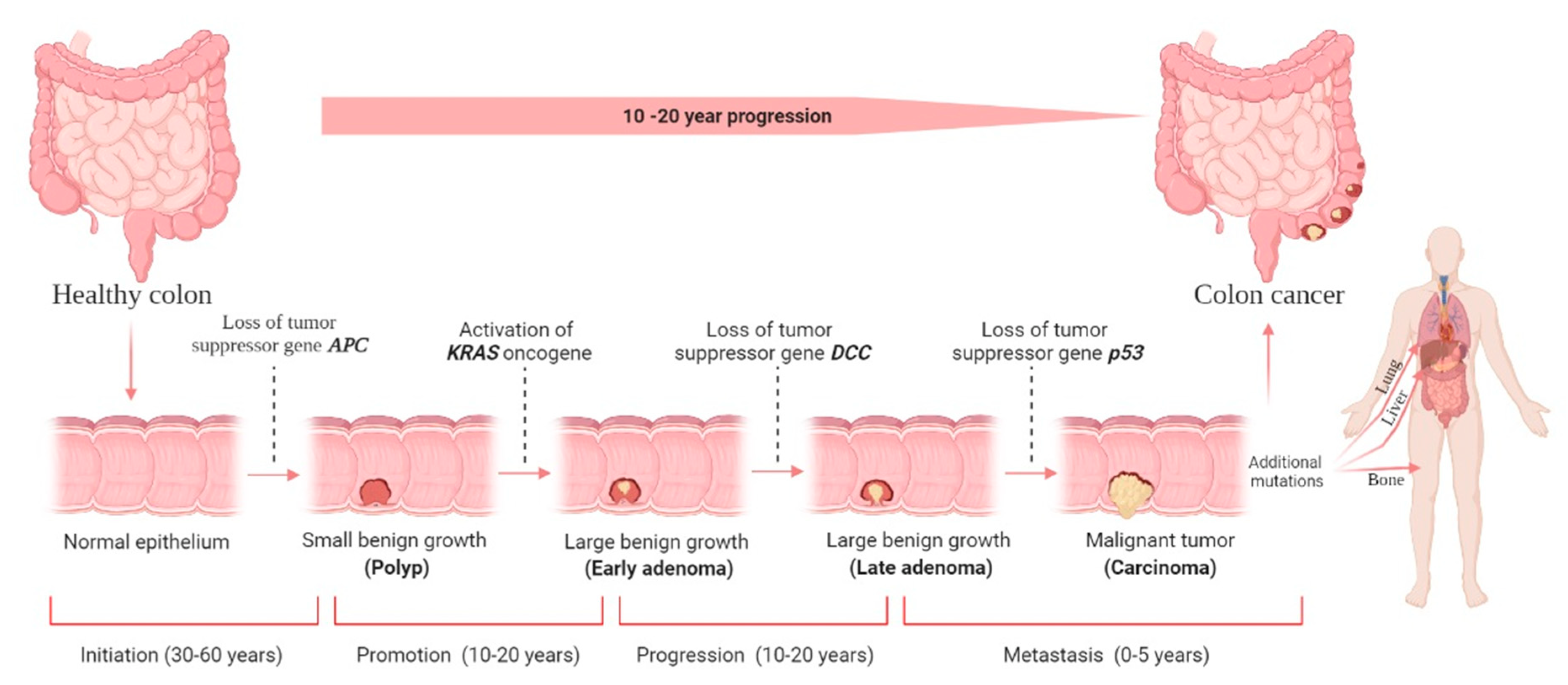 Figure 1. Colorectal cancer (CRC) stages and development. There are four stages in the development of CRC carcinogenesis: initiation, promotion, progression, and metastasis. The liver is the most common metastatic site, followed by the lung and bone. Although it is difficult to determine the duration required for each stage, decades will likely be required to form CRC. The figure was created using BioRender.com (accessed on 30 December 2021).
Figure 1. Colorectal cancer (CRC) stages and development. There are four stages in the development of CRC carcinogenesis: initiation, promotion, progression, and metastasis. The liver is the most common metastatic site, followed by the lung and bone. Although it is difficult to determine the duration required for each stage, decades will likely be required to form CRC. The figure was created using BioRender.com (accessed on 30 December 2021).Usually, a non-cancerous development results in dysplastic tissue formation (tumor), leading to CRC development once the cells have undergone several abnormal DNA changes. A non-cancerous (benign) soft tissue tumor is a growth that does not spread (metastasize) to other parts of the body. Hyperproliferation causes a (benign) polyp or adenoma to form (stage 0). Ten percent of adenomatous polyps can become malignant, forming an adenocarcinoma that invades the muscularis propria (stage I). The tumor grows in volume and further invades tissue in the serosa (stage II) and visceral peritoneum (stage III). Then, there is the possibility of developing lymphatic or blood vessel metastasis (stage IV) [22]. The stage determines the severity of the disease and the therapy options available [23]. Even though surgery is the standard treatment choice for stages 0–II CRC, stage III CRC requires surgery and adjuvant chemotherapy, and stage IV and recurrent CRC require surgery, chemotherapy, and targeted therapy; however, unfortunately, it has no known certainty established cure until now.
3. Current Global Epidemiology of Colorectal Cancer
According to GLOBOCAN data, in 2020, there were an estimated 19.3 million new cases and 10 million cancer deaths worldwide, of which CRC contributed about 1.93 million (10%) further incidences and 0.94 million (9.4%) deaths (Figure 2a,b). The overall estimated age-standardized global distribution of incidence and mortality of CRC for both sexes and all ages is shown in Figure 3. The incidence and mortality of CRC vary considerably between countries and among world regions. They are also associated with the socioeconomic status of the country. According to the World Bank, the new cases and deaths are more remarkable in areas with higher income levels and lesser in areas with lower income levels.
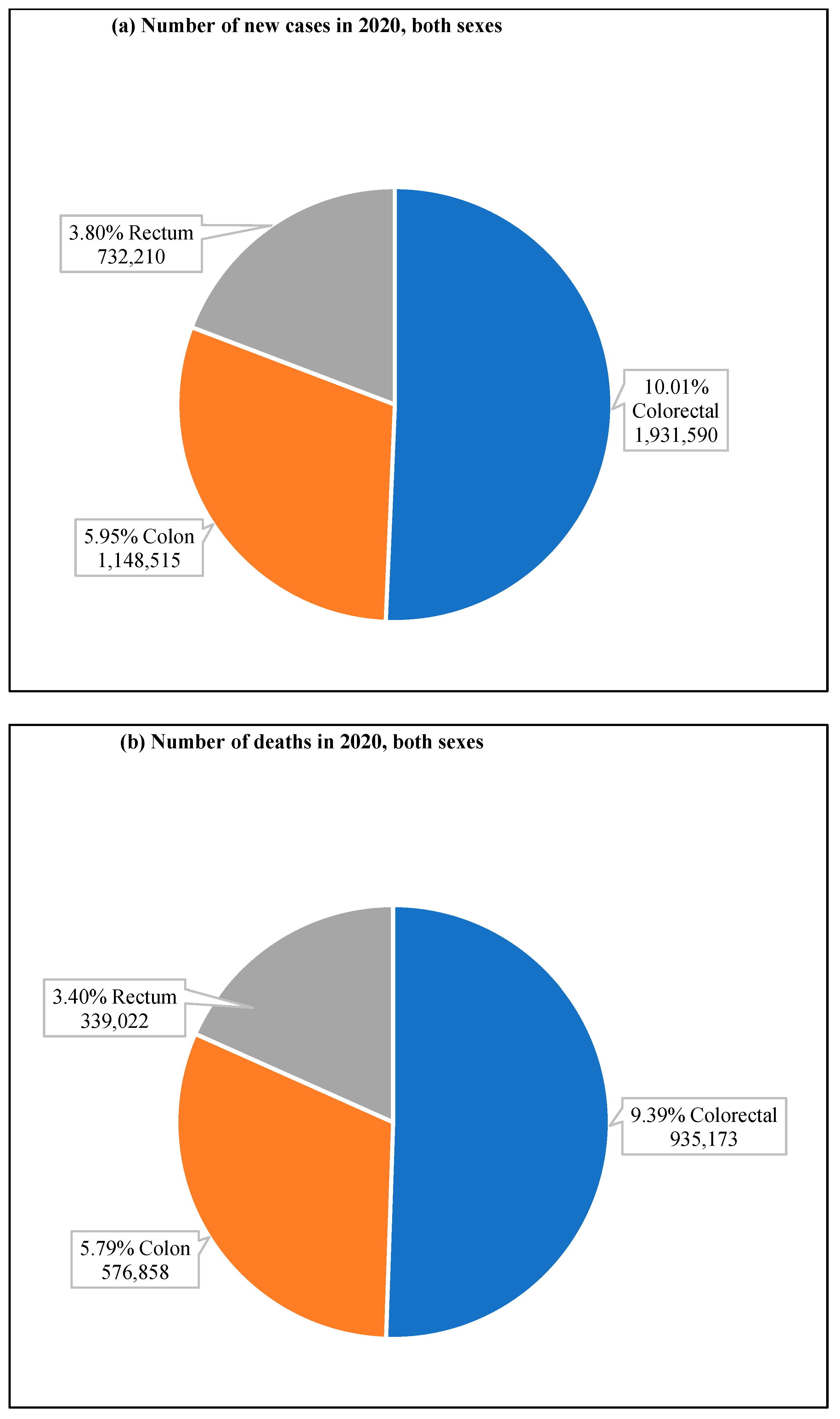 Figure 2. CRC new cases and deaths in 2020. (a) shows new cases, both sexes and all ages, and (b) shows deaths of both sexes for all age groups. The value shown in % is calculated against the total number of all cancers. The data source is GLOBOCAN [24], taken with permission.
Figure 2. CRC new cases and deaths in 2020. (a) shows new cases, both sexes and all ages, and (b) shows deaths of both sexes for all age groups. The value shown in % is calculated against the total number of all cancers. The data source is GLOBOCAN [24], taken with permission.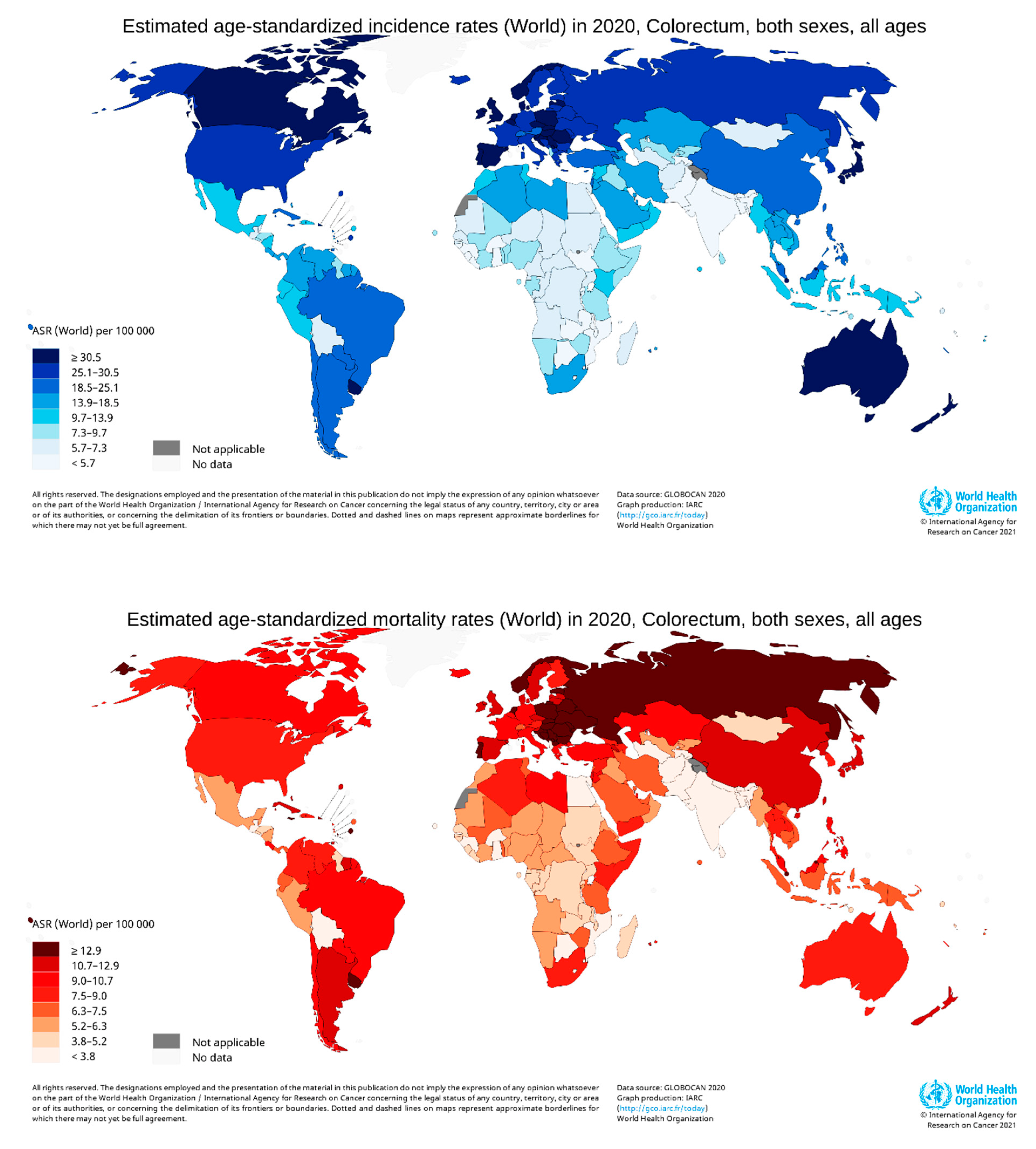 Figure 3. Map showing the global distribution of estimated age-standardized incidence rates (top) and mortality rate (bottom) of CRC in 2020 for both sexes and all ages. (Reproduced from GLOBOCAN [24] with permission).
Figure 3. Map showing the global distribution of estimated age-standardized incidence rates (top) and mortality rate (bottom) of CRC in 2020 for both sexes and all ages. (Reproduced from GLOBOCAN [24] with permission).The incidence and deaths of CRC are increasing in developing countries for both males and females, even though it is significantly high in high-income countries. The upper-middle-income countries accounted for the highest incidences (45.94%) and deaths (49.37%). The higher-income countries recorded fewer incidences (42.43%) than upper-middle-income countries; however, the deaths were significantly lower (36.40%), perhaps due to better treatment facilities (Figure 4). The high-income and upper-middle-income countries covered more than 88 and 85% of incidence and mortality, respectively (Table 1) [25].
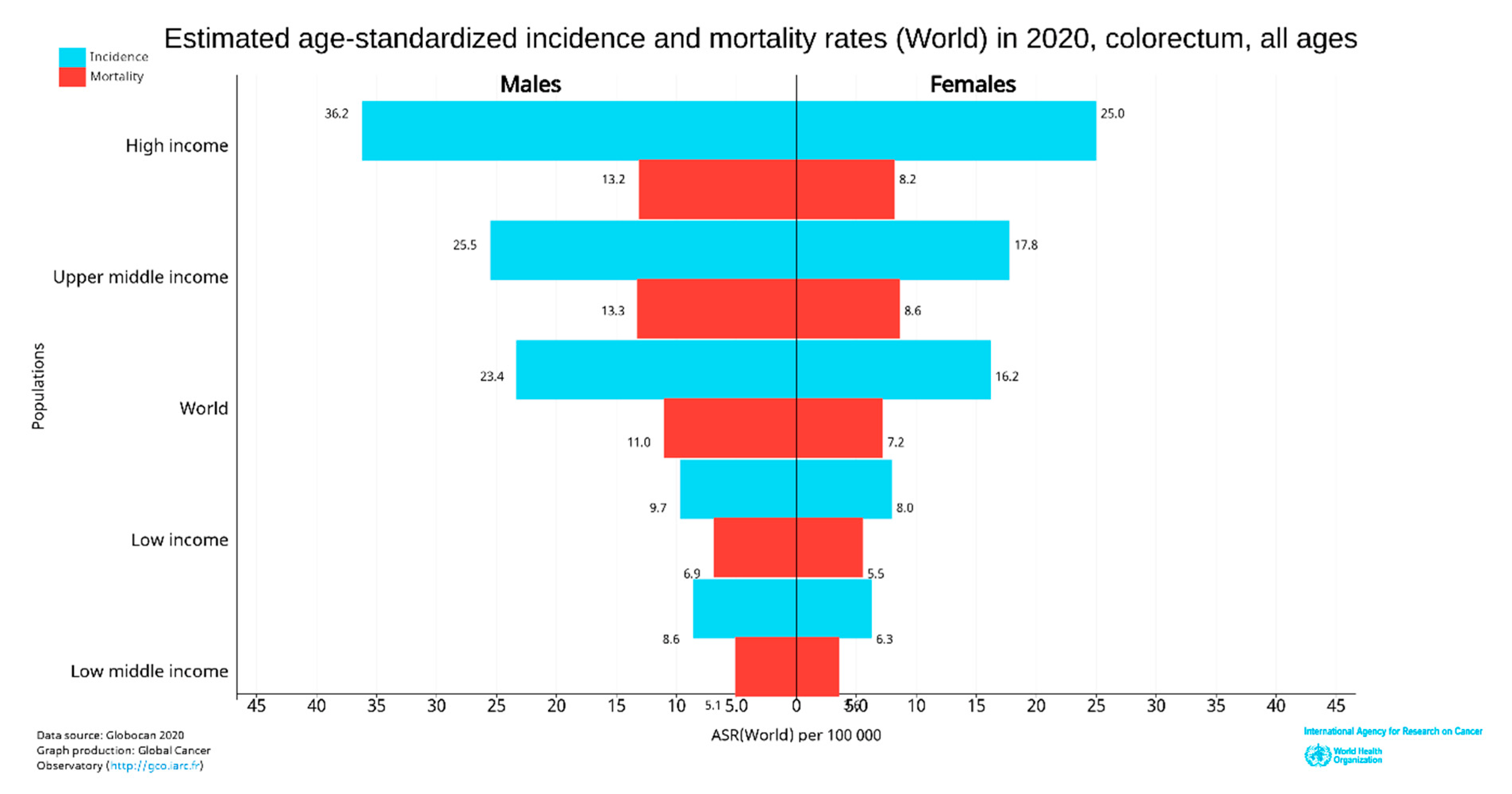 Figure 4. World CRC incidence and mortality rates in 2020 (according to income level for all age groups). The data were extracted from GLOBOCAN [24] with permission.
Figure 4. World CRC incidence and mortality rates in 2020 (according to income level for all age groups). The data were extracted from GLOBOCAN [24] with permission.Table 1. World CRC estimated age-standardized incidence and mortality rates in 2020 (all ages).
| Population * | Incidence (%) | Mortality (%) |
|---|---|---|
| Upper middle income | 887,025 (45.94) | 461,511 (49.37) |
| High income | 819,143 (42.43) | 340,272 (36.40) |
| Low middle income | 194,954 (10.10) | 112,556 (12.04) |
| Low income | 29,542 (1.53) | 20,392 (2.18) |
| Total | 1,930,664 | 934,731 |
* Data extracted from GLOBOCAN [24] with permission.
In 2020, CRC was the most diagnosed cancer (out of 36 cancers) among men in 18 of the 186 countries worldwide and women in 6 of the 185 countries [25]. However, in 2018, CRC was the most diagnosed among men in 10 of 185 countries, and no country had CRC as the most diagnosed cancer among women [26]. So, the CRC incidence rate has increased to about 10 from 5% in the last two years in men. Women were predominant in 3.24% of countries (Figure 4). CRC is more common among men than women and more than four times more common in high-income countries than low-income countries. The deaths were also about 2.5 times higher in high-income nations than in low-income nations.
In 2020, age-standardized (world) incidence rates per 100,000 of CRC in both sexes were 19.8, with males having a higher incidence rate of 23.4 and females with 16.2, which is almost equal to the incidence of 2018 [25][26]. The death rate in both sexes was 9.1; for men was 11, and for women was 7.2 per 100,000 CRC (Figure 4).
Among CRC, colon cancer was predominant and accounted for 59.5% of new cases, 61.9% of deaths and rectum cancer had 37.9% of incidence and 36.3% mortality for both sexes and all ages (Figure 5). Colon cancer alone stands fifth for new cancer cases and deaths compared to all cancers. In contrast, rectum cancer is the eighth and tenth most severe cancer for incidences and mortality, respectively [25].
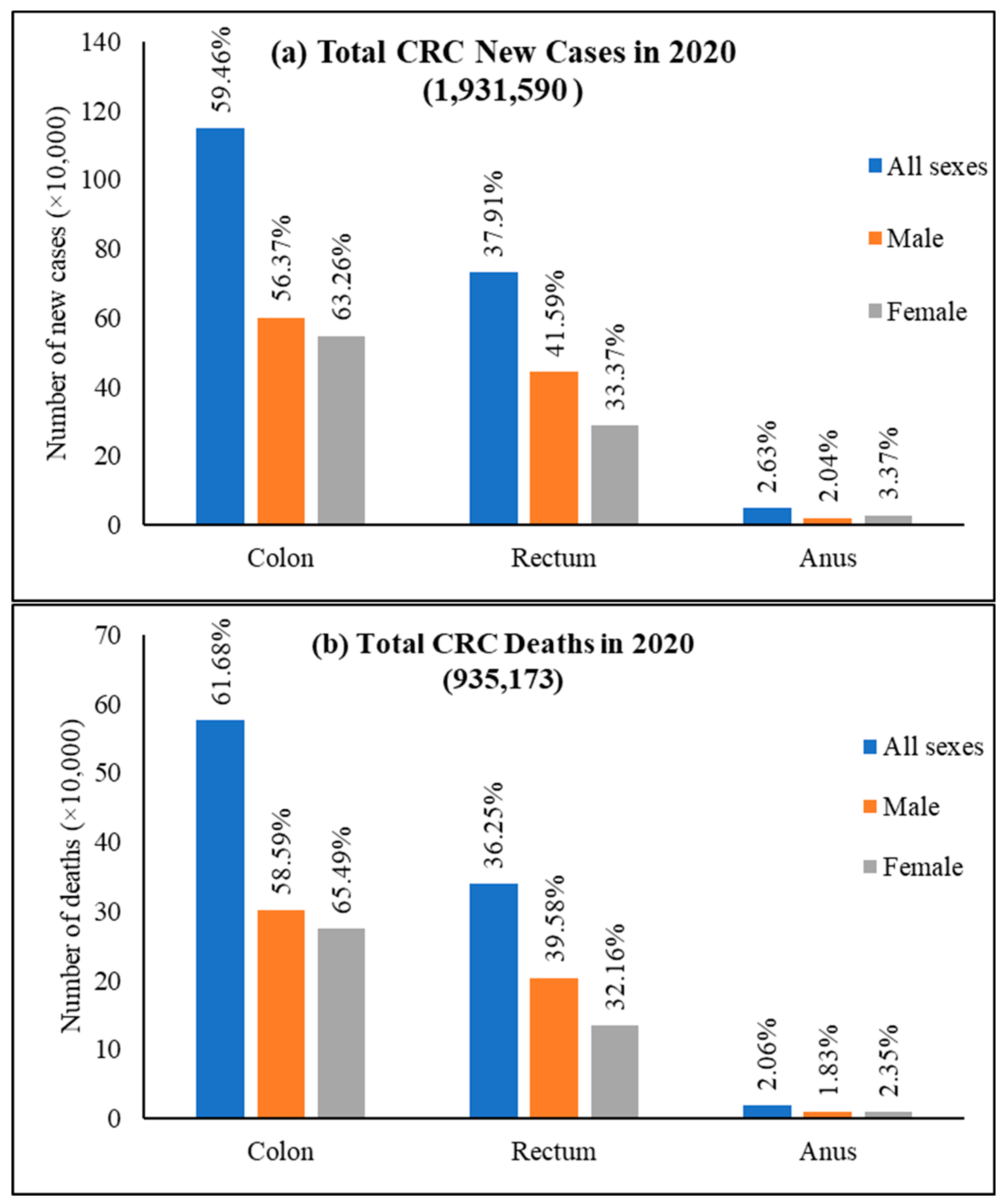 Figure 5. World new cases and deaths of colon cancer, rectum cancer, and anus cancer in 2020. (a) New cases and (b) deaths of colon cancer, rectum cancer, and anus cancer. The value is shown in % on top of each column and is calculated against the CRC number in 2020 for both sexes and all ages. Data extracted from GLOBOCAN [24] with permission.
Figure 5. World new cases and deaths of colon cancer, rectum cancer, and anus cancer in 2020. (a) New cases and (b) deaths of colon cancer, rectum cancer, and anus cancer. The value is shown in % on top of each column and is calculated against the CRC number in 2020 for both sexes and all ages. Data extracted from GLOBOCAN [24] with permission.This entry is adapted from the peer-reviewed paper 10.3390/cancers14071732
This entry is offline, you can click here to edit this entry!
