Nanoplastics are defined as particles with a size ranging between 1 nm and 1 μm, which is different from the classification criteria of engineered nanomaterials (ENMs). The bioeffects of nanoplastics should be fully explored owing to their potentially enhanced toxicity and small size.
- nanoplastics
- human health
- hazard identification
1. Introduction
2. Another Two Sources of Nanoplastics in the Environment
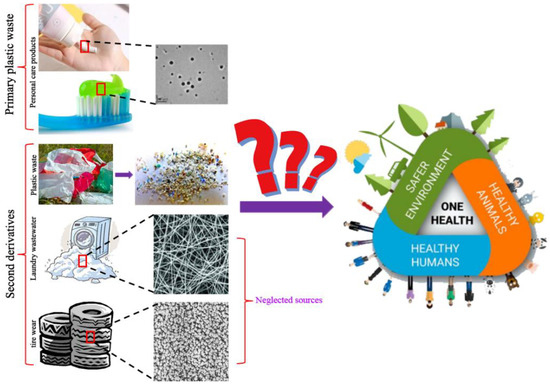
2.1. Nanoplastics from Tire Wear
2.2. Nanoplastics from Laundry Wastewater
3. Potential Exposure Routes of Nanoplastics and Adverse Effects on Humans
3.1. Potential Exposure Routes of Nanoplastics to Humans
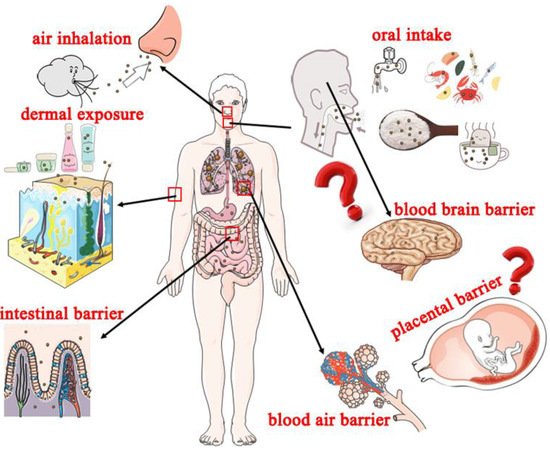
3.1.1. Oral Intake
3.1.2. Air Inhalation
3.1.3. Dermal Exposure
3.2. Potential Adverse Effects of Nanoplastics on Human
4. Behavior of Nanoplastics
4.1. Interactions with Biological Media
4.2. Interactions with Cell Membrane
4.2.1. Cell Internalization
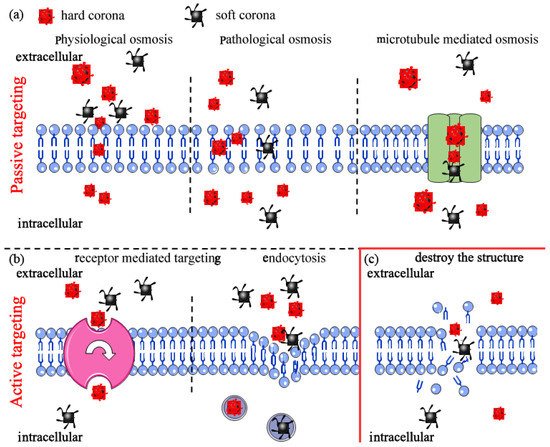
4.2.2. Nanoplastics Destroy Cell Membrane Structure Leading to Cell Death
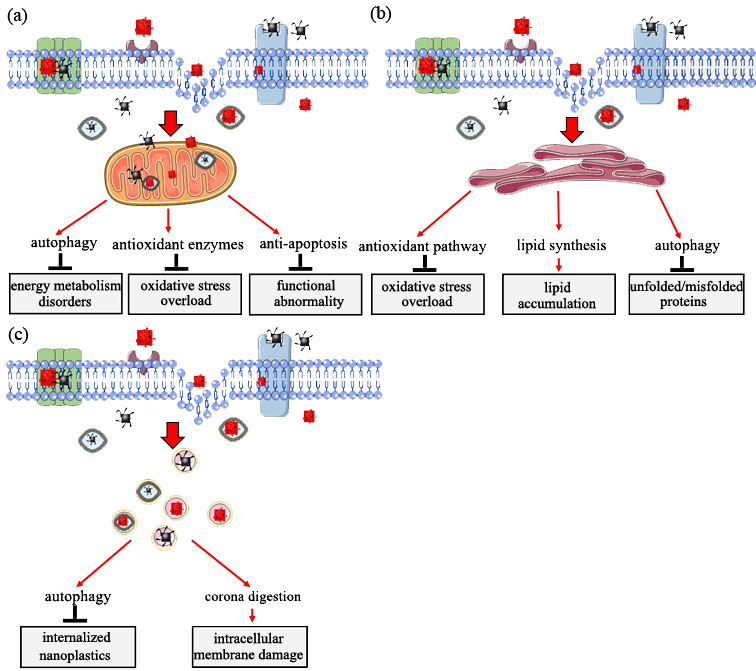
Figure 4. Target organelle toxicity induced by nanoplastics: (a) role of mitochondria in response to nanoplastics toxicity; (b) role of endoplasmic reticulum in response to nanoplastics toxicity; (c) role of lysosome in response to nanoplastics toxicity.
5. Target Organelle Toxicity Induced by Nanoplastics
In vivo and in vitro experiments indicate that nanoplastics penetrate cell membranes and are internalized into cells, inducing intracellular biological effects [47,70–72]. Mitochondria, endoplasmic reticulum, and lysosome play vital roles in response to nanoplastics toxicity [70,73]. The subsequent sections present the main functions of the three organelles in eukaryotic cells in response to nanoplastic exposure.
5.1. Role of Mitochondria in Response to Nanoplastic Toxicity
Mitochondria is the major site for cell energy supply and oxidative phosphorylation. Exposure of mitochondria to external stimuli, such as nanomaterials, affects its normal structure and function, leading to metabolic and functional disorders [74]. Findings from previous studies indicate that internalized nanoparticles, including nanoplastics, are targeted to the mitochondria [70,75].
A previous study explored human bronchial epithelial BEAS-2B cells exposed to nanoplastics, and the results showed no significant morphological changes, such as swollen mitochondria. However, significant functional changes, such as abnormal energy metabolism, were observed in the mitochondria and the specific performances [32]. Organisms have a self-protection mechanism (enhanced autophagy) of oxidative mitochondrial activity that occurs to supply enough energy for regular homeostasis [71]. A study using zebrafish as the model animal showed that nanoplastics alter mitochondrial function by increasing oxygen consumption (OCR) from five aspects (rate basal, maximum, nonmitochondrial, basal mitochondrial, and mitochondrial spare capacity) in female gonad cells [47]. In addition, cells of Sterechinus neumayeri initiate a crucial self-protection mechanism of oxidative mitochondrial activity mediated by upregulation of superoxide dismutase (SOD), catalase (CAT), and metallothionein (MT) expression to maintain permeabilization of the mitochondrial membrane and activation of anti-apoptotic signaling of Bcl-2-caspase-8 after exposure to nanoplastics [76]. Moreover, a recent study reported the role of an anti-apoptotic-signaling cascade (Bcl2-Apaf1-caspase3) in response to nanoplastics using the C. elegans model. The study explored the upstream-signaling cascade of DNA damage (HUS1/Tel2p-p53-BH3), which exhibited the important self-protection strategy of the mitochondria in the regulation of nanoplastics toxicity [68]. These findings indicate that the mitochondria exhibit defensive mechanisms in response to nanomaterials toxicity, especially toxicity from nanoplastics (Figure 4a).
5.2. Role of Endoplasmic Reticulum in Response to Nanoplastics Toxicity
The endoplasmic reticulum is a subcellular organelle widely distributed in the cytoplasm of almost all eukaryotic cells. It is an important site for protein and lipid synthesis and plays a key role in intracellular signal transduction implicated in cell survival and apoptosis [77–79]. Studies have not fully explored whether nanoplastic particles penetrate the endoplasmic reticulum.
The imbalance of endoplasmic reticulum homeostasis occurs when organisms are under physiological or pathological stimulation. This imbalance leads to the accumulation of unfolded or misfolded proteins or changes in Ca2+ concentration in the endoplasmic reticulum lumen and ultimately induces unfolded protein response [77,80]. Significant upregulation of Grp78 and Grp170 expression is observed after exposure of coelomocytes to nanoplastics, indicating that exposure to nanoplastics induces pathways for oxidative stress alleviation and stress-related autophagy in endoplasmic reticulum [76]. Long-term exposure to nanoplastics at low doses causes endoplasmic reticulum stress, unfolded protein response, and fat metabolism disorder in intestinal cells of C. elegans. These effects are modulated through activation and phosphorylation of intracellular mitogen-activated protein kinase 14 (MAPK14), resulting in the upregulation of X-box binding protein 1 (XBP1). These proteins induce the endoplasmic reticulum unfolded protein response and dysregulation of sterol regulatory element-binding transcription factor 2 (SREBF2) and mediator complex subunit 15 (MED15). Subsequently, dysregulation of these factors affects lipid accumulation and modulate stearoyl-CoA desaturase (SCD) and stearoyl-CoA desaturase 5 (SCD5), ultimately inducing an innate immune response [71,81]. In addition, expression activating transcription factor 6 (ATF6), DDIT3 (DNA damage-inducible transcript 3 protein) and ERN1 (endoplasmic reticulum to nucleus signaling 1) is upregulated, inducing expression of immunofluorescence assay of microtubule-associated protein 1 light chain 3 (LC3-II) and accumulation of autophagosomes in bronchial epithelial BEAS-2B cells after exposure to nanoplastics. These changes indicate a potential autophagy regulation mechanism through the ER stress caused by misfolded protein aggregation [70]. These findings indicate that the endoplasmic reticulum is a crucial subcellular structure in response to the biological effects of nanoplastics (Figure 4b).
5.3. Role of Lysosome in Response to Nanoplastics Toxicity
The lysosome is an intracellular digestive organelle and the site for enzyme activities involved in removal of pathological cellular waste. Lysosomes can fuse with autophagosomes to form auto-phagolysosome in which lysosomal proteases degrade engulfed components [15,82,83].
Previous studies reported that nanoplastics internalized in eukaryotic cells accumulate in lysosomes. The accumulation of induced changes in lysosomal PH and membrane integrity ultimately cause lysosomal dysfunction [23,84]. Moreover, accumulation of nanoplastics induce the autophagic response through the activation of transcription factor, EB (TFEB), which further promotes an increase in lysosome–autophagosome fusion and, ultimately, enhances clearance of autophagic cargo [82]. Notably, overall blockage of autophagic flux if not alleviated can ultimately result in cell death due to severe damage of lysosomes [23]. A previous study reported that nanoplastics are translocated into lysosomes through a self-protection mechanism called the ‘Trojan horse’ effect. In this case, nanoplastics are covered by a layer of either soft or hard corona under a biological microenvironment, and intracellular membrane damage occurs once the surface of the corona is degraded [85]. Studies should further evaluate the effects of nanoplastics in lysosomes (Figure 4c).
6. Challenges and Future Research
The findings summarized in this review indicate that further studies should explore sources and effects of microplastics and nanoplastics. A summary of the aspects that should be evaluated is provided below.
6.1. Detection of Nanoplastics in the Environment
Although we have lessons from engineered nanomaterial research, existing analytical techniques are not still sufficiently developed to quantify nanoplastics in the environment, especially in biological samples. Previous studies report some methods used for the detection of nanoplastics, such as asymmetric flow field-flow fractionation coupled to multi-angle light scattering, fluorescent labeled, and Raman tweezers [86–89], but some issues still remain to be addressed. For example, fluorescent labeled, as the most frequently used detection method, particularly in cellular bioaccumulation, often involves artifacts, leading to false positives [88,90]. Considering that dye leakage and cellular autofluorescence might be the main sources of artifacts, dye core-wrapped and blank negative control should be used to alleviate the problem [88]. Given the discussion above, more efficient, convenient, and accurate analysis methods should be developed and applied to identify nanoplastics in the environment. Meanwhile, a complete set of detection systems for microplastics and nanoplastics in different media should be established to alleviate exposure of human to these plastic particles.
6.2. Elimination or Reduction of Microplastic or Nanoplastic Pollution
The separation and collection of nanoplastics from the environment is a challenge; however, there are some potential methods for reducing the levels of nanoplastics.
6.2.1. Recycling
Approximately 6300 Mt of plastic waste was generated in 2015 [91]. More than 90% (5733 Mt) of plastic waste produced in 2015 was not recycled and were directly or indirectly released to the environment, and the level is projected to be 12,000 in 2050 [92]. Plastic waste released to the environment may eventually be degraded to microsize or nanosize; thus, recycling of plastic waste is an effective way to eliminate or reduce micropollutions or nanopollutions.
6.2.2. Substitute Materials
Two types of materials can be used as substitutes for plastics. Chitin is a bioactive polymer widely used in industrial and biomedical fields. It is one of the most abundant natural polysaccharides [93]. Chitin has unique properties, such as high antibiosis activity, non-toxicity, ease of chemical synthesis and modification, and high biodegradability; therefore, it is a feasible substitute material for plastics [94]. Hemp fiber is a biodegradable polymer material widely used in the manufacture of ropes, automobile parts, polystyrene, and elastic building materials [95,96]. Hemp fiber is biodegradable, recyclable, and nontoxic; thus, it is a potential substitute for plastics.
6.2.3. Degradation of Microplastic or Nanoplastic Pollutions
Degradation of environmental pollutants, including microplastics and nanoplastics, is conducted using physical, chemical, or biological methods. Previous studies show that chemical and physical methods are used to eliminate or reduce micropollutions or nanopollutions; however, these methods lead to the production of new pollutants or are associated with incomplete degradation [97,98]. Biodegradation can be applied to overcome the limitations of the traditional methods of degradation of pollutants [99]. Biodegradation is highly effective and has less side effects, thus playing a vital role in elimination or reduction of microplastic or nanoplastic pollutions from the environment.
6.3. Comprehensive Analysis of Nanoplastics Toxicity
6.3.1. Toxicity of Aged Nanoplastics and Their Leachings
Nanoplastics released to the environment can result in absorption and leaching of environmental chemicals during their transport and transformation in different media [88,100]. Aged nanoplastics release high amounts of additives into the environment. In addition, their properties can be altered, increasing the potential toxicity. However, the effects of nanoplastics to ecology and humans have not been fully elucidated. Therefore, studies should explore the chemicals released from nanoplastics to the environment and their potential effects.
6.3.2. Toxicity of Nanoplastics at Environmentally Relevant Concentrations (ERC)
Most studies expose nanoplastics to some model organisms at concentrations unlikely to exist in the real environment [61,100]. Few studies have explored the toxicity of nanoplastics at environmentally relevant concentrations (ERC). Therefore, studies should explore the effects of nanoplastics at the concentrations that they exist in the environment.
Several animal models have been used to explore various toxicity types induced by nanoplastics, such as reproductive toxicity, neuronal toxicity, and developmental toxicity. However, studies have not fully explored the potential toxicity of nanoplastics on humans [68,101,102]. Ineluctable exposure of nanoplastics to humans further drive the need to explore the effects of these particles on humans.
7. Conclusions
In this review, uncommon sources of nanoplastics, such as tire wear and laundry wastewater, were summarized in the present study to evaluate the relationship between nanoplastics and human health. The findings indicate that the potential sources of clothing and tire wear may result in high amounts of microplastics or nanoplastics in the environment. Further, the potential exposure routes, such as oral, inhaled, or dermal exposure, and the long-term biological effects of nanoplastics, such as crossing biological barriers and generation-crossing, were explored. In addition, the biointerface of nanoplastics was evaluated and the latent paths of entry into eukaryotic cells, including passive targeting and active targeting, were summarized. Furthermore, the effects of nanoplastic particles on intracellular target organelles, with mitochondria, endoplasmic reticulum, and lysosome as examples, were explored to describe the role of organelles in response to nanoplastic toxicity. These findings provide information on exposure of nanoplastics and the potential biological effects.
Author Contributions: Conception and design: M.Q. and H.L.; investigation: H.L. and X.L.; writing—original draft preparation: M.Q. All authors have read and agreed to the published version of the manuscript.
Funding: We acknowledge the financial support from the Jiangsu Province Innovative Talents (No. 337090129), Yangzhou Green Yang Jinfeng Project (No. 137012416) and National Natural Science Foundation Youth Fund (No. 81903357).
Data Availability Statement: No new data were created or analyzed in this review.
Conflicts of Interest: There are no conflicts of interest to declare.
References
- Lebordais, M.; Gutierrez-Villagomez, J.M.; Gigault, J.; Baudrimont, M.; Langlois, V.S. Molecular impacts of dietary exposure to nanoplastics combined with arsenic in Canadian oysters (Crassostrea virginica) and bioaccumulation comparison with Caribbean oysters (Isognomon alatus). Chemosphere 2021, 277, 130331.
- Zhang, Z.; Gao, S.H.; Luo, G.; Kang, Y.; Zhang, L.; Pan, Y.; Zhou, X.; Fan, L.; Liang, B.; Wang, A. The contamination of microplastics in China’s aquatic environment: Occurrence, detection and implications for ecological risk. Pollut. 2022, 296, 118737.
- Prata, J.C.; da Costa, J.P.; Lopes, I.; Duarte, A.C.; Rocha-Santos, T. Environmental exposure to microplastics: An overview on possible human health effects. Total Environ. 2020, 702, 134455.
- Ragusa, A.; Svelato, A.; Santacroce, C.; Catalano, P.; Notarstefano, V.; Carnevali, O.; Papa, F.; Rongioletti, M.C.A.; Baiocco, F.; Draghi, S.; et al. Plasticenta: First evidence of microplastics in human placenta. Int. 2021, 146, 106274.
- Hirt, N.; Body-Malapel, M. Immunotoxicity and intestinal effects of nano- and microplastics: A review of the literature. Fibre Toxicol. 2020, 17, 57.
- Sussarellu, R.; Suquet, M.; Thomas, Y.; Lambert, C.; Fabioux, C.; Pernet, M.E.J.; Le Goic, N.; Quillien, V.; Mingant, C.; Epelboin, Y.; et al. Oyster reproduction is affected by exposure to polystyrene microplastics. Natl. Acad. Sci. USA 2016, 113, 2430–2435.
- Dawson, A.L.; Kawaguchi, S.; King, C.K.; Townsend, K.A.; King, R.; Huston, W.M.; Nash, S.M.B. Turning microplastics into nanoplastics through digestive fragmentation by Antarctic krill. Commun. 2018, 9, 1001.
- Hartmann, N.B.; Hüffer, T.; Thompson, R.C.; Hassellöv, M.; Verschoor, A.; Daugaard, A.E.; Rist, S.; Karlsson, T.; Brennholt, N.; Cole, M.; et al. Are we speaking the same language? Recommendations for a definition and categorization framework for plastic debris. Sci. Technol. 2019, 53, 1039–1047.
- Gigault, J.; El Hadri, H.; Nguyen, B.; Grassl, B.; Rowenczyk, L.; Tufenkji, N.; Feng, S.Y.; Wiesner, M. Nanoplastics are neither microplastics nor engineered nanoparticles. Nanotechnol. 2021, 16, 501–507.
- Gigault, J.; Halle, A.T.; Baudrimont, M.; Pascal, P.Y.; Gauffre, F.; Phi, T.L.; El Hadri, H.; Grassl, B.; Reynaud, S. Current opinion: What is a nanoplastic? Pollut. 2018, 235, 1030–1034.
- Revel, M.; Chatel, A.; Mouneyrac, C. Micro(nano)plastics: A threat to human health? Opin. Environ. Sci. Health 2018, 1, 17–23.
- Mintenig, S.M.; Loder, M.G.J.; Primpke, S.; Gerdts, G. Low numbers of microplastics detected in drinking water from ground water sources. Total Environ. 2019, 648, 631–635.
- Jemec, A.; Horvat, P.; Kunej, U.; Bele, M.; Krzan, A. Uptake and effects of microplastic textile fibers on freshwater crustacean Daphnia magna. Pollut. 2016, 219, 201–209.
- Dalela, M.; Shrivastav, T.G.; Kharbanda, S.; Singh, H. pH-Sensitive Biocompatible nanoparticles of paclitaxel-conjugated poly(styrene-co-maleic acid) for anticancer drug delivery in solid tumors of syngeneic mice. ACS Appl. Mater. Inter. 2015, 7, 26530–26548.
- Wang, L.; Xie, X.J.; Cao, T.C.; Bosset, J.; Bakker, E. Surface-doped polystyrene microsensors containing lipophilic solvatochromic dye transducers. Eur. J. 2018, 24, 7921–7925.
- Hernandez, L.M.; Yousefi, N.; Tufenkji, N. Are there nanoplastics in your personal care products? Sci. Technol. Let. 2017, 4, 280–285.
- Song, Y.K.; Hong, S.H.; Eo, S.; Jang, M.; Han, G.M.; Isobe, A.; Shim, W.J. Horizontal and vertical distribution of microplastics in korean coastal waters. Sci. Technol. 2018, 52, 12188–12197.
- Zhang, G.S.; Liu, Y.F. The distribution of microplastics in soil aggregate fractions in southwestern China. Total Environ. 2018, 642, 12–20.
- Gasperi, J.; Wright, S.L.; Dris, R.; Collard, F.; Mandin, C.; Guerrouache, M.; Langlois, V.; Kelly, F.J.; Tassin, B. Microplastics in air: Are we breathing it in? Opin. Environ. Sci. Health 2018, 1, 1–5.
- Yang, D.; Shi, H.; Li, L.; Li, J.; Jabeen, K.; Kolandhasamy, P. Microplastic pollution in table salts from China. Sci. Technol. 2015, 49, 13622–13627.
- Peng, L.; Fu, D.; Qi, H.; Lan, C.Q.; Yu, H.; Ge, C. Micro- and nano-plastics in marine environment: Source, distribution and threats—A review. Total Environ. 2020, 698, 134254.
- Zhang, K.; Su, J.; Xiong, X.; Wu, X.; Wu, C.; Liu, J. Microplastic pollution of lakeshore sediments from remote lakes in Tibet plateau, China. Pollut. 2016, 219, 450–455.
- Wang, F.J.; Salvati, A.; Boya, P. Lysosome-dependent cell death and deregulated autophagy induced by amine-modified polystyrene nanoparticles. Open Biol. 2018, 8, 170271.
- Wik, A.; Dave, G. Occurrence and effects of tire wear particles in the environment—A critical review and an initial risk assessment. Pollut. 2009, 157, 1–11.
- Kreider, M.L.; Panko, J.M.; McAtee, B.L.; Sweet, L.I.; Finley, B.L. Physical and chemical characterization of tire-related particles: Comparison of particles generated using different methodologies. Total Environ. 2010, 408, 652–659.
- Sharma, K.M. Traffic generated non-exhaust particulate emissions from concrete pavement: A mass and particle size study for two-wheelers and small cars. Environ. 2009, 43, 5691–5697.
- Dahl, A.; Gharibi, A.; Swietlicki, E.; Gudmundsson, A.; Bohgard, M.; Ljungman, A.; Blomqvist, G.; Gustafsson, M. Traffic-generated emissions of ultrafine particles from pavement-tire interface. Environ. 2006, 40, 1314–1323.
- Mathissen, M.; Scheer, V.; Vogt, R.; Benter, T. Investigation on the potential generation of ultrafine particles from the tire–road interface. Environ. 2011, 45, 6172–6179.
- Leads, R.R.; Weinstein, J.E. Occurrence of tire wear particles and other microplastics within the tributaries of the Charleston Harbor Estuary, South Carolina, USA. Pollut. Bull. 2019, 145, 569–582.
- Dris, R.; Gasperi, J.; Mirande, C.; Mandin, C.; Guerrouache, M.; Langlois, V.; Tassin, B. A first overview of textile fibers, including microplastics, in indoor and outdoor environments. Pollut. 2017, 221, 453–458.
- Hernandez, L.M.; Xu, E.G.; Larsson, H.; Rui, T.; Tufenkji, N. Plastic teabags release billions of microparticles and nanoparticles into tea. Sci. Technol. 2019, 53, 12300–12310.
- Carney Almroth, B.M.; Åström, L.; Roslund, S.; Petersson, H.; Johansson, M.; Persson, N.K. Quantifying shedding of synthetic fibers from textiles; a source of microplastics released into the environment. Sci. Pollut. Res. Int. 2018, 25, 1191–1199.
- Sillanpää, M.; Sainio, P. Release of polyester and cotton fibers from textiles in machine washings. Sci. Pollut. Res. Int. 2017, 24, 19313–19321.
- Lenz, R.; Enders, K.; Nielsen, T.G. Microplastic exposure studies should be environmentally realistic. Natl. Acad. Sci. USA 2016, 113, E4121–E4122.
- Galloway, T. Micro- and Nano-Plastics and Human Health; Springer: Cham, Switzerland,
- Alimi, O.S.; Farner Budarz, J.; Hernandez, L.M.; Tufenkji, N. Microplastics and nanoplastics in aquatic environments: Aggregation, deposition, and enhanced contaminant transport. Sci. Technol. 2018, 52, 1704–1724.
- Al-Sid-Cheikh, M.; Rowland, S.J.; Stevenson, K.; Rouleau, C.; Henry, T.B.; Thompson, R.C. Uptake, whole-body distribution, and depuration of nanoplastics by the Scallop Pecten maximus at environmentally realistic concentrations. Sci. Technol. 2018, 52, 14480–14486.
- Xu, Y.; He, Q.; Liu, C.; Huangfu, X. Are micro- or nanoplastics leached from drinking water distribution systems? Sci. Technol. 2019, 53, 9339–9340.
- Cox, K.D.; Covernton, G.A.; Davies, H.L.; Dower, J.F.; Juanes, F.; Dudas, S.E. Human consumption of microplastics. Sci. Technol. 2019, 53, 7068–7074.
- Winkler, A.; Santo, N.; Ortenzi, M.A.; Bolzoni, E.; Bacchetta, R.; Tremolada, P. Does mechanical stress cause microplastic release from plastic water bottles? Water Res. 2019, 166, 115082.
- Hoogenboom, L.A.P.; Hoogenboom, L.A.P.; Hoogenboom, L.A.P. Statement: Presence of microplastics and nanoplastics in food, with particular focus on seafood. EFSA J. 2016, 14, e04501.
- Yee, S.L.; Hii, L.W.; Looi, C.K.; Lim, W.M.; Leong, C.O. Impact of microplastics and nanoplastics on human health. Nanomaterials 2021, 11, 496.
- Hong, S.H.; Shim, W.J.; Hong, L. Methods of analysing chemicals associated with microplastics: A review. Methods 2017, 9, 1361–1368.
- Kole, P.J.; Lhr, A.J.; Belleghem, F.G.A.J.V.; Ragas, A.M.J. Wear and tear of tyres in the global environment: Size distribution, emission, pathways and health effects. In Proceedings of the SETAC Europe 29th Annual Meeting, Helsinki, Finland, 26–30 May 2019.
- Vogt, A.; Combadiere, B.; Hadam, S.; Stieler, K.M.; Lademann, J.; Schaefer, H.; Autran, B.; Sterry, W.; Blume-Peytavi, U. 40 nm, but not 750 or 1,500 nm, nanoparticles enter epidermal CD1a+ cells after transcutaneous application on human skin. Invest. Dermatol. 2006, 126, 1316–1322.
- Schneider, M.; Stracke, F.; Hansen, S.; Schaefer, U.F. Nanoparticles and their interactions with the dermal barrier. Dermatoendocrinology 2009, 1, 197–206.
- Kozal; Jordan, S.; Levin; Edward, D.; Pitt; Jordan, A.; Di, G.; Richard, T.; Trevisan; Rafael. Maternal transfer of nanoplastics to offspring in zebrafish (Danio rerio): A case study with nanopolystyrene. Total Environ. 2018, 643, 324–334.
- Lehner, R.; Petri-Fink, A.; Rothen-Rutishauser, B. Nanoplastic impact on human health—A 3D intestinal model to study the interaction with nanoplastic particles. In Proceedings of the International Conference on Microplastic Pollution in the Mediterranean Sea; Springer Water: Capri, Italy, 26-29, Sep 2017.
- Philipp; Schwabl; Sebastian; Kppel; Knigshofer; Theresa; Bucsics; Michael; Trauner; Thomas. Detection of various microplastics in human stool: A prospective case series. Intern. Med. 2019, 171, 453–457.
- Tagesson, C.; Sjödahl, R. Passage of molecules through the wall of the gastrointestinal tract. J. Gastroenterol. 1978, 13, 519–524.
- Gehr, P.; Bachofen, M.; Weibel, E.R. The normal human lung: Ultrastructure and morphometric estimation of diffusion capacity. Physiol. 1978, 32, 121–140.
- Yang, C.S.; Chang, C.H.; Tsai, P.J.; Chen, W.Y.; Tseng, F.G.; Lo, L.W. Nanoparticle-based in vivo investigation on blood-brain barrier permeability following ischemia and reperfusion. Chem. 2004, 76, 4465–4471.
- Mattsson, K.; Johnson, E.V.; Malmendal, A.; Linse, S.; Hansson, L.A.; Cedervall, T. Brain damage and behavioural disorders in fish induced by plastic nanoparticles delivered through the food chain. Rep. 2017, 7, 11452.
- Grafmueller, S.; Manser, P.; Diener, L.; Diener, P.A.; Maeder-Althaus, X.; Maurizi, L.; Jochum, W.; Krug, H.F.; Buerki-Thurnherr, T.; von Mandach, U.; et al. Bidirectional transfer study of polystyrene nanoparticles across the placental barrier in an ex vivo human placental perfusion model. Health Perspect. 2015, 123, 1280–1286.
- Francia, V.; Yang, K.; Deville, S.; Reker-Smit, C.; Nelissen, I.; Salvati, A. Corona composition can affect the mechanisms cells use to internalize nanoparticles. ACS Nano 2019, 13, 11107–11121.
- Dzuricky, M.; Xiong, S.; Weber, P.; Chilkoti, A. Avidity and cell uptake of integrin-targeting polypeptide micelles is strongly shape-dependent. Nano Lett. 2019, 19, 6124–6132.
- Kihara, S.; Ghosh, S.; McDougall, D.R.; Whitten, A.E.; Mata, J.P.; Köper, I.; McGillivray, D.J. Structure of soft and hard protein corona around polystyrene nanoplastics-particle size and protein types. Biointerphases 2020, 15, 051002.
- Kihara, S.; van der Heijden, N.J.; Seal, C.K.; Mata, J.P.; Whitten, A.E.; Köper, I.; McGillivray, D.J. Soft and Hard interactions between polystyrene nanoplastics and human serum albumin protein corona. Bioconjugate Chem. 2019, 30, 1067–1076.
- Baimanov, D.; Cai, R.; Chen, C. Understanding the chemical nature of nanoparticle-protein interactions. Bioconjugate Chem. 2019, 30, 1923–1937.
- Xu, M.; Halimu, G.; Zhang, Q.; Song, Y.; Fu, X.; Li, Y.; Li, Y.; Zhang, H. Internalization and toxicity: A preliminary study of effects of nanoplastic particles on human lung epithelial cell. Total Environ. 2019, 694, 133794.
- Lee, W.S.; Cho, H.J.; Kim, E.; Huh, Y.H.; Kim, H.J.; Kim, B.; Kang, T.; Lee, J.S.; Jeong, J. Bioaccumulation of polystyrene nanoplastics and their effect on the toxicity of Au ions in zebrafish embryos. Nanoscale 2019, 11, 3173–3185.
- Bhushan, B.; Khanadeev, V.; Khlebtsov, B.; Khlebtsov, N.; Gopinath, P. Impact of albumin based approaches in nanomedicine: Imaging, targeting and drug delivery. Colloid Interfac. 2017, 246, 13–39.
- Montel, L.; Pinon, L.; Fattaccioli, J. A Multiparametric and high-throughput assay to quantify the influence of target size on phagocytosis. J. 2019, 117, 408–419.
- dos Santos, T.; Varela, J.; Lynch, I.; Salvati, A.; Dawson, K.A. Quantitative assessment of the comparative nanoparticle-uptake efficiency of a range of cell lines. Small 2011, 7, 3341–3349.
- Hollóczki, O.; Gehrke, S. Can nanoplastics alter cell membranes? ChemPhysChem 2020, 21, 9–12.
- Elliott, J.T.; Rösslein, M.; Song, N.W.; Toman, B.; Kinsner-Ovaskainen, A.; Maniratanachote, R.; Salit, M.L.; Petersen, E.J.; Sequeira, F.; Romsos, E.L.; et al. Toward achieving harmonization in a nano-cytotoxicity assay measurement through an interlaboratory comparison study. Altex 2017, 34, 201–218.
- Hong, S.; Leroueil, P.R.; Janus, E.K.; Peters, J.L.; Kober, M.M.; Islam, M.T.; Orr, B.G.; Baker, J.R., Jr.; Banaszak Holl, M.M. Interaction of polycationic polymers with supported lipid bilayers and cells: Nanoscale hole formation and enhanced membrane permeability. Bioconjugate Chem. 2006, 17, 728–734.
- Qu, M.; Qiu, Y.; Kong, Y.; Wang, D. Amino modification enhances reproductive toxicity of nanopolystyrene on gonad development and reproductive capacity in nematode Caenorhabditis elegans. Pollut. 2019, 254, 112978.
- Pinsino, A.; Bergami, E.; Della Torre, C.; Vannuccini, M.L.; Addis, P.; Secci, M.; Dawson, K.A.; Matranga, V.; Corsi, I. Amino-modified polystyrene nanoparticles affect signalling pathways of the sea urchin (Paracentrotus lividus) embryos. Nanotoxicology 2017, 11, 201–209.
- Lim, S.L.; Ng, C.T.; Zou, L.; Lu, Y.; Chen, J.; Bay, B.H.; Shen, H.M.; Ong, C.N. Targeted metabolomics reveals differential biological effects of nanoplastics and nanoZnO in human lung cells. Nanotoxicology 2019, 13, 1117–1132.
- Qu, M.; Liu, Y.; Xu, K.; Wang, D. Activation of p38 MAPK signaling-mediated endoplasmic reticulum unfolded protein response by nanopolystyrene particles. Biosys. 2019, 3, e1800325.
- Jin, Y.; Lu, L.; Tu, W.; Luo, T.; Fu, Z. Impacts of polystyrene microplastic on the gut barrier, microbiota and metabolism of mice. Total Environ. 2019, 649, 308–317.
- Reinholz, J.; Diesler, C.; Schöttler, S.; Kokkinopoulou, M.; Ritz, S.; Landfester, K.; Mailänder, V. Protein machineries defining pathways of nanocarrier exocytosis and transcytosis. Acta Biomater. 2018, 71, 432–443.
- Armstrong, J.S. Mitochondrial medicine: Pharmacological targeting of mitochondria in disease. J. Pharmacol. 2007, 151, 1154–1165.
- Li, Y.; Wu, Q.; Zhao, Y.; Bai, Y.; Chen, P.; Xia, T.; Wang, D. Response of microRNAs to in vitro treatment with graphene oxide. ACS Nano 2014, 8, 2100–2110.
- Bergami, E.; Emerenciano, A.K.; Gonzalez-Aravena, M.; Cardenas, C.A.; Hernandez, P.; Silva, J.; Corsi, I. Polystyrene nanoparticles affect the innate immune system of the Antarctic sea urchin Sterechinus neumayeri. Polar Biol. 2019, 42, 743–757.
- Schwarz, D.S.; Blower, M.D. The endoplasmic reticulum: Structure, function and response to cellular signaling. Mol. Life Sci. 2016, 73, 79–94.
- Marciniak, S.J. Endoplasmic reticulum stress in lung disease. Respir. Rev. 2017, 26, 170018.
- Wang, M.; Kaufman, R.J. Protein misfolding in the endoplasmic reticulum as a conduit to human disease. Nature 2016, 529, 326–335.
- Ron, D.; Walter, P. Signal integration in the endoplasmic reticulum unfolded protein response. Rev. Mol. Cell Bio. 2007, 8, 519–529.
- Yang, Y.; Shao, H.; Wu, Q.; Wang, D. Lipid metabolic response to polystyrene particles in nematode Caenorhabditis elegans. Pollut. 2020, 256, 113439.
- Song, W.; Popp, L.; Yang, J.; Kumar, A.; Gangoli, V.S.; Segatori, L. The autophagic response to polystyrene nanoparticles is mediated by transcription factor EB and depends on surface charge. Nanobiotechnol. 2015, 13, 87.
- Saftig, P.; Haas, A. Turn up the lysosome. Cell Biol. 2016, 18, 1025–1027.
- Fröhlich, E.; Meindl, C.; Roblegg, E.; Ebner, B.; Absenger, M.; Pieber, T.R. Action of polystyrene nanoparticles of different sizes on lysosomal function and integrity. Fibre Toxicol. 2012, 9, 26.
- Wang, F.; Yu, L.; Monopoli, M.P.; Sandin, P.; Mahon, E.; Salvati, A.; Dawson, K.A. The biomolecular corona is retained during nanoparticle uptake and protects the cells from the damage induced by cationic nanoparticles until degraded in the lysosomes. Nanomedicine 2013, 9, 1159–1168.
- Correia, M.; Loeschner, K. Detection of nanoplastics in food by asymmetric flow field-flow fractionation coupled to multi-angle light scattering: Possibilities, challenges and analytical limitations. Bioanal. Chem. 2018, 410, 5603–5615.
- Gagné, F. Detection of polystyrene nanoplastics in biological tissues with a fluorescent molecular rotor probe. Xenobiot. 2019, 9, 8147.
- Catarino, A.I.; Frutos, A.; Henry, T.B. Use of fluorescent-labelled nanoplastics (NPs) to demonstrate NP absorption is inconclusive without adequate controls. Total Environ. 2019, 670, 915–920.
- Gillibert, R.; Balakrishnan, G.; Deshoules, Q.; Tardivel, M.; Magazzù, A.; Donato, M.G.; Maragò, O.M.; Lamy de La Chapelle, M.; Colas, F.; Lagarde, F.; et al. Raman tweezers for small microplastics and nanoplastics identification in seawater. Sci. Technol. 2019, 53, 9003–9013.
- ScSchür, C.; Rist, S.; Baun, A.; Mayer, P.; Hartmann, N.B.; Wagner, M. When fluorescence is not a particle: The tissue translocation of microplastics in Daphnia magna seems an artifact. Toxicol. Chem. 2019, 38, 1495–1503.
- Andrady, A.L. Plastics and the Environment; John Wiley Sons: Hoboken, NJ, USA, 2003; Volume 51, pp. 23–30.
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Adv. 2017, 3, e1700782.
- Hosseinnejad, M.; Jafari, S.M. Evaluation of different factors affecting antimicrobial properties of chitosan. J. Biol. Macromol. 2016, 85, 467–475.
- Muxika, A.; Etxabide, A.; Uranga, J.; Guerrero, P.; de la Caba, K. Chitosan as a bioactive polymer: Processing, properties and applications. J. Biol. Macromol. 2017, 105, 1358–1368.
- Mazzanti, V.; Pariante, R.; Bonanno, A.; Ballesteros, O.D.; Mollica, F.; Filippone, G. Reinforcing mechanisms of natural fibers in green composites: Role of fibers morphology in a PLA/hemp model system. Sci. Technol. 2019, 180, 51–59.
- Sepe, R.; Bollino, F.; Boccarusso, L.; Caputo, F. Influence of chemical treatments on mechanical properties of hemp fiber reinforced composites. Part B Eng. 2017, 133, 210–217.
- Jian, K.; Li, Z.; Xiaoguang, D.; Hongqi, S.; Zhimin, A. Degradation of cosmetic microplastics via functionalized carbon nanosprings. Matter 2019, 1, 745–758.
- Day, M.; Cooney, J.D.; Klein, C.; Fox, J. Thermal Degradation of Automotive Plastics: A Possible Recycling Opportunity; ACS Publications: Washington, DC, USA,
- Ding, Q.; Liu, K.; Xu, K.; Sun, R.; Zhang, J.; Yin, L.; Pu, Y. Further understanding of degradation pathways of microcystin-lr by an indigenous Sphingopyxis sp. in environmentally relevant pollution concentrations. Toxins 2018, 10, 536.
- Luo, H.; Xiang, Y.; He, D.; Li, Y.; Zhao, Y.; Wang, S.; Pan, X. Leaching behavior of fluorescent additives from microplastics and the toxicity of leachate to Chlorella vulgaris. Total Environ. 2019, 678, 1–9.
- Qu, M.; Kong, Y.; Yuan, Y.; Wang, D. Neuronal damage induced by nanopolystyrene particles in nematode Caenorhabditis elegans. Sci. Nano 2019, 6, 2591–2601.
- Tallec, K.; Huvet, A.; Di Poi, C.; Gonzalez-Fernandez, C.; Lambert, C.; Petton, B.; Le Goic, N.; Berchel, M.; Soudant, P.; Paul-Pont, I. Nanoplastics impaired oyster free living stages, gametes and embryos. Pollut. 2018, 242, 1226–1235.
This entry is adapted from the peer-reviewed paper 10.3390/nano12081298
