The perturbation of the diversity and proportions of species within the oral microbiota leads to dysbiosis and associated increased risk of local and systemic diseases. In children who receive chemotherapy for cancer, oral mucositis is a common and painful side effect that decreases quality of life (QoL) and treatment adherence. The oral microbiota undergoes a substantial dysbiosis as an effect of cancer and its treatment, characterized by lower richness and less diversity. Furthermore, this dysbiosis seems to promote pro-inflammatory cytokine release and pro-apoptotic mediators, enhancing the oral tissue damage. Further studies on the role of the oral microbiota in the pathogenesis of oral mucositis should be performed among children with cancer who receive chemotherapy, to find preventive and protective factors against the pathogenesis of oral mucositis.
1. Introduction
The human oral cavity is a major gateway to the human body, harboring the second most abundant microbiota after the gastrointestinal tract. The oral microbiota is constituted by the microorganisms found in the human oral cavity (that includes teeth, gingival sulcus, attached gingiva, tongue, cheek, lip, hard and soft palate) and its contiguous extensions (such as tonsils, pharynx, esophagus, Eustachian tube, middle ear, trachea, lungs, nasal passages and sinuses) until the distal esophagus [1].
The oral microbiota is crucial for keeping homeostasis in the oral cavity. The perturbation of the diversity and proportions of species within the microbiota, with a single or few species predomination, leads to dysbiosis and associated increased risk of local and systemic diseases
[2].
Childhood is a chief time for the founding of the adult oral microbiota. In fact, the shaping of an individual’s oral microbiota may be influenced by several factors (genetic determinants, pregnancy term, delivery mode, and feeding method) that occur in early childhood. Furthermore, specific changes occur in oral microbiota diversity in several healthy pediatric conditions (early childhood caries, pediatric obesity, celiac disease, autism, Henoch-Shonlein purpura disease, pediatric appendicitis, pediatric inflammatory bowel diseases, pediatric hemato-oncological diseases), as described in various cross-sectional and case-control studies
[3]. The analysis of the oral microbiota in various clinical conditions allows one to draw a specific disease risk profile for each patient and to set treatment options as personalized as possible
[4][5]. Pediatric patients with cancer are a peculiar set of patients, in which the study of the oral microbiota may provide important information about the prognosis and the most common illness-related complications, such as chemotherapy-induced mucositis
[6].
2. Physiological and Pathological Changes of the Oral Microbiota during Childhood
The colonization of the oral cavity by microorganisms takes place from intrauterine life (through amniotic fluid, uterine membranes and meconium) and after birth it is never stable, but continuously shaped by several influencing factors, such as pregnancy term, delivery mode, and feeding method
[7][8][9].
Premature newborns may be hospitalized in a neonatal intensive care unit (NICU) for a variable time, thus they may be colonized by environmental microbes, including commensal and pathogenic microorganisms, such as
Streptococcus,
Staphylococcus,
Neisseria and
Enterobacteriaceae (usually found on continuous positive airway pressure machines, stethoscopes, ventilators, incubators, radiant warmers),
Geobacillus,
Halomonas,
Shewanella,
Acinetobacter, and
Gemella (discovered on computer screens, a computer mouse, freezer handles, hand sanitizer bottles, door handles, telephones, heart rate monitor screens, alarm buttons)
[10].
The type of delivery (vaginal delivery or cesarean section) seems to influence the composition of the oral microbiota only in the first week after birth. Newborns born from vaginal delivery have early colonization by bacteria present in the vaginal canal, such as
Lactobacilli,
Prevotella,
Bacterioides, while those born by caesarean section show early colonization by
Veillonella,
Corynebacterium,
Propionibacterium and
Staphylococci. However, in both cases, a preponderance of Streptococci may be observed with the initiation of breastfeeding
[7].
During the lactation period, there is observed a predominance of Gram-positive bacteria, like
Streptococcus,
Granulicatella and
Veillonella.
Streptococci are especially present in breast milk and they are very able to adhere to mucous membranes
[11].
Then the main changes in the oral microbiota occur between 6 and 24 months
[12], with the consequent transition from a predominance of
Streptococcus mutans,
Fusobacteria,
Tenericutes,
TM7, and
SR1. With the appearance of primary dentition, specific niches colonized by different bacterial species are observed; furthermore, oral bacteria diversity continues to grow through time, with about 550 OTUs at the end of primary dentition
[11]. With the appearance of the definitive dentition, the oral microbiota of each child can be defined as personalized. The oral microbiota of adults is more influenced by oral hygiene habits, while in children of the same familiar nucleus, oral hygiene seems to not affect the diversity of bacterial species
[13].
Among children, a significant difference in bacterial species of the oral cavity is related to weight and lifestyle. Burcham et al. found that obese children and those with a BMI near to obesity have an oral microbiota poorer in bacterial species than non-obese children
[13].
More recently the relationship between the microenvironment of the oral cavity, oral pathologies and systemic diseases have been widely analyzed. The proposed mechanisms of these oral-systemic links include the spread of bacteria from the oral cavity in the form of bacteremia or circulating bacterial toxins, which may trigger an increase of circulating pro-inflammatory cytokines and a weakness of the immune system, favoring systemic diseases or their complications
[14][15][16].
The association between dysbiosis and the onset of neoplasms was already investigated in oncologic patients. In patients with cancer, the microbial host homeostasis may be disrupted by cancer itself, by the bactericidal effect of chemotherapy and antibacterial agents adopted against secondary infections, but also by the immunodeficiency induced by chemotherapy
[17].
Although a direct causality has not yet been proven, individual members of the oral microbiome may promote tumorigenesis and may be adopted as potential biomarkers in several types of adult cancer. As examples of specific organisms as biomarkers, an increase in
Fusobacterium nucleatum has been linked to colorectal cancer,
Porphyromonas gingivalis and
Fusobacterium spp. have been linked to pancreatic cancer
[18].
3. Oral microbiota and Chemotherapy-Induced Oral Mucositis in Children
Common side effects associated with the treatment of pediatric cancers are oral mucositis, which may produce pain, difficulty feeding, malnutrition, prolonged hospitalization and potential bloodstream infection, inducing a significant decline in patients’ quality of life (QoL) and compliance to the treatment
[19][20].
The incidence rate of oral mucositis ranges from 52% in patients who receive standard chemotherapy to 100% in patients who receive high-dose chemotherapy. If not managed with adequate measures, oral mucositis represents an important limiting factor of chemotherapy and may worsen patient prognosis and compliance
[21]. Malnutrition, pre-existing medical conditions, alterations in salivary production and composition, and poor oral health have been reported as patient-associated risk factors for the development of oral mucositis
[22][23].
Saliva is the first barrier for defending against microbial invasion through mechanical, nonimmunologic and immunologic functions. Its continuous flow eliminates food debris and exogenous harmful factors. Salivary immunoglobulins and high-molecular-weight glycoproteins, restrain bacterial metabolism. A group of salivary proteins (such as lysozyme, peroxidase, myeloperoxidase, lactoferrin), together with other salivary components (thiocyanate, chlorine, hydrogen peroxide), are able to interfere with the growth of oral bacteria and fungi. Karoleweska et al. analyzed the salivary flow of 44 children with ALL, finding that the introduction of chemotherapy caused a decrease in salivary secretion rate and S-IgA concentration. Furthermore, patients who developed oral mucositis presented lower myeloperoxidase and peroxidase concentration than those without oral mucositis
[24]. Hedge et al. found a deterioration in oral health status and gingival status, with increased dental caries in the group of analyzed children who received chemotherapy for ALL.
The inflammation of the oral mucosa observed in pediatric oral mucositis may be scored using the World Health Organization (WHO) system, which grades the oral damage into five stages that occur consecutively and are mechanistically linked (
grade 0: no change;
grade 1: soreness/erythema;
grade 2: erythema, ulcers, can eat solids;
grade 3: ulcers can eat liquid diet only;
grade 4: oral alimentation not possible)
[25].
The initial injury of the mucosa membranes occurs concurrently with chemotherapy or radiotherapy administration. The type of chemotherapeutic agents, their dosage and the schedule of administration are important factors affecting the severity of the mucosal injury. Chemotherapeutic drugs associated with an important risk of mucositis are alkylating agents (busulfan, cyclophosphamide, procarbazine, thiotepa), anthracyclines (daunorubicin, doxorubicin, and epirubicin), platinum compounds (cisplatin, carboplatin, oxaliplatin), antimetabolite agents (cytosine arabinoside, hydroxyurea, 5-flurouracil, methotrexate, 6-mercaptopurine, and 6-thioguanide), antibiotics (actinomycin D, bleomycin, mitomycin), vinca alkaloids (vinblastin and vincristine) and taxanes (docetaxel). Melphalan, doxorubicin, 5-fluoruracil, methotrexate, and cisplatin etoposide may produce a high stomatotoxic effect, directly causing mucosal breakdown
[26].
Correct oral hygiene and a good gingival condition are associated with a lesser incidence of oral mucositis. Basic oral care involves multi-agent combination oral care protocols based on daily oral hygiene with a soft toothbrush and toothpaste, mouthwash with sodium bicarbonate and chlorhexidine. Few therapeutic options are effective for prevention and treatment of oral mucositis and many of them are still being studied. Antioxidant agents (amifostine, glutamine, oral zinc supplement, vitamin E, N-acetyl-cysteine, GC4419), inhibitors of cytokines production (turmeric, clonidine lauriad buccal tablets, pentoxifylline, dusquetide), natural agents (honey, aloe vera gel, chamomile mouthwash, MF5232), oral cryotherapy, and probiotics are currently under investigation for mucositis prevention
[27]. Moreover, treatment of pain associated with mucositis should be obtained with opioids, such as morphine. Local mouthwashes with chlorhexidine and 0.2% morphine may allow better pain control than systemic analgesic treatment
[28].
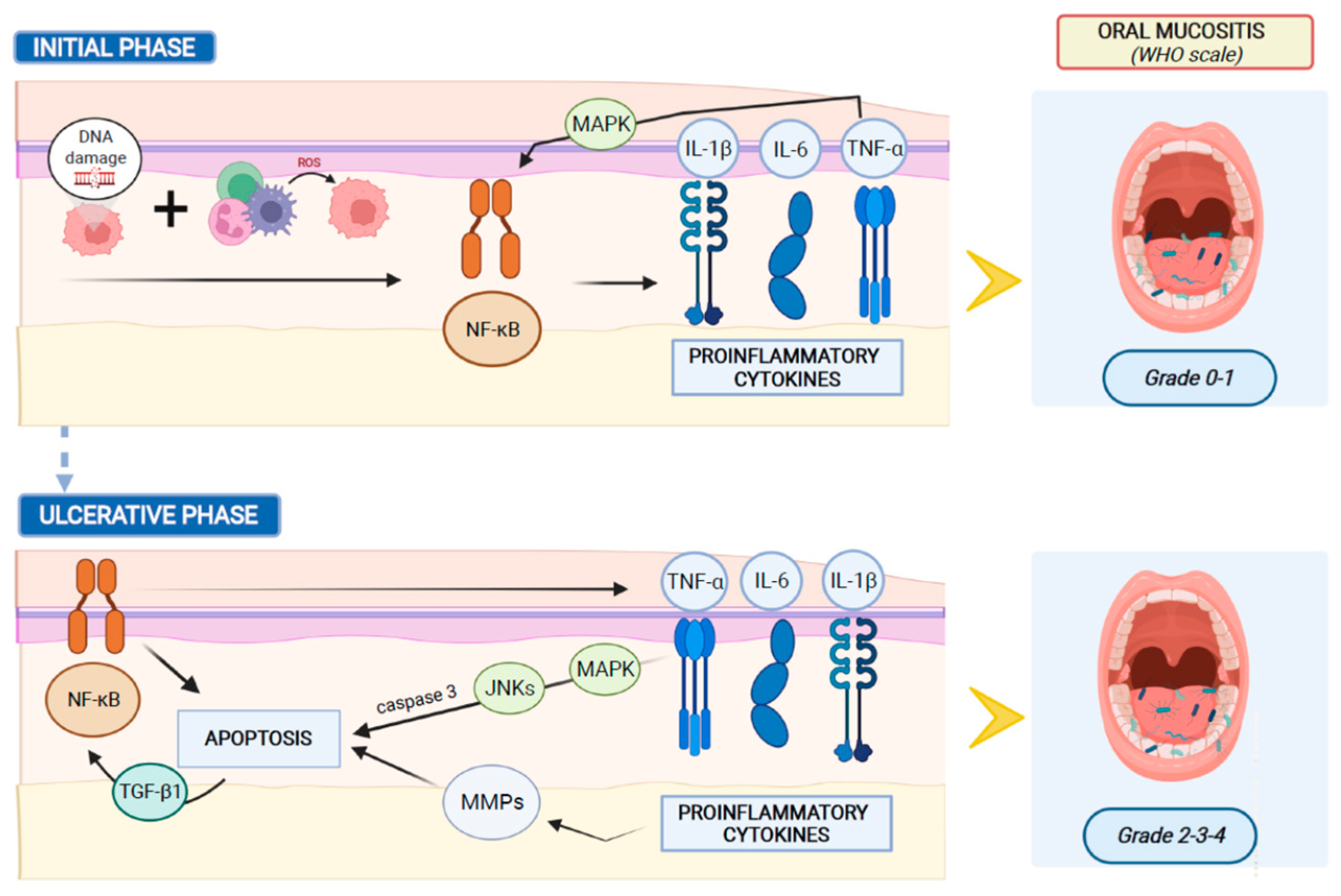
In humans, the development of oral mucositis consists of a cascade of events that occur consecutively and are automatically linked.
Systemic chemotherapy induces tissue damage causing reactive oxygen species (ROS) release and DNA damage, leading to cell death of the basal and suprabasal epithelial cells. DNA strand breaks lead to the activation of the apoptotic process, which is regulated by p53 activation and increased caspase 3 and endogenous damage-associated pattern molecules (DAMPs)
[29]. The response to this initial damage characterizes the second stage of mucositis development. During this stage the cells of the injured mucosa promote the transcription of several genes involved in the mucositis process. In this molecular scenario, nuclear factor-κB (NF-κB) represents the main transcriptional mediator that modulates over 200 genes associated with proinflammatory cytokines, such as tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), and interleukin-1β (IL-1β). Pro-inflammatory cytokines colonize oral mucosa, inducing early damage of connective tissue and endothelium, inhibiting tissue oxygenation and promoting epithelial basal cell death
[30]. Simultaneously with the activation of other pathways, the primary damage is amplified through a positive feedback loop mechanism. The released TNF-α initiates the activation of the mitogen-activated protein kinase (MAPK) on target cells and sustains NF-κB activity. During this stage, despite the impairment of mucosa and sub-mucosa, patients usually exhibit few symptoms without macroscopic evidence of mucosal injury.
Afterward, MAPK signaling mediates caspase 3 activation and cell death through the activation of c-Jun N-terminal Kinases (JNKs), which modify Activator Protein 1 (AP1) transcriptional activity. Moreover, the high levels of TNF-α and IL-1β produce an amplification of the pro-apoptotic signal, through the activation of Matrix Metalloproteinase (MMP) activity. Furthermore, the injured keratinocytes release Transforming Growth Factor-beta 1 (TGF-β1), which inhibits the cell cycle, recruits leucocytes and sustains NF-κB activity, improving the damage-mediated signaling
[31][32].
Figure 1 shows the main mechanisms of oral mucositis pathogenesis.
Figure 1. Main mechanisms of oral mucositis pathogenesis.
Clinical manifestations of mucositis are noticeable at the fourth stage of the inflammation process. During this stage, the mucosa and sub-mucosa are ulcerated, patients complain of pain and oral nutrition is not possible. Furthermore, the breaks in the submucosa promote bacterial translocation, which may explain the involvement of oral microbiota in the pathogenesis of oral mucositis. Symbiotic inhabitant microorganisms of the healthy mucosa invade the damaged submucosa, leading to mononuclear infiltrating cell-mediated inflammation response, which promotes new pro-inflammatory cytokine release, amplifies the expression of pro-apoptotic mediators, and increases the tissue damage
[33].
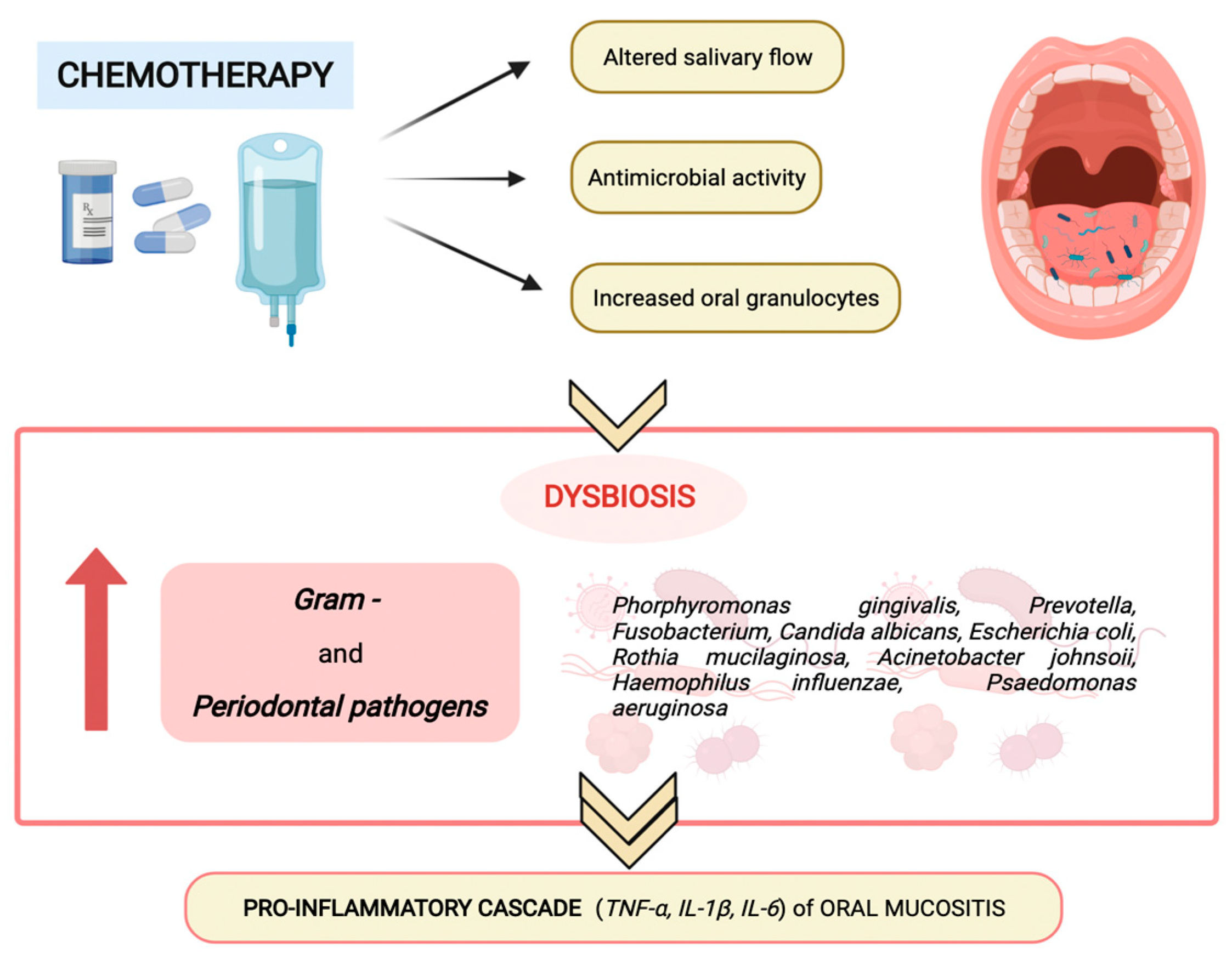
The altered salivary production and composition, the augmented oral granulocyte presence and the antimicrobial activity of chemotherapy modify oral microbiota composition and homeostasis, favoring the predominance of Gram-negative anaerobes and periodontal pathogens (as shown in
Figure 2), such as
Porphyromonas gingivalis,
Fusobacterium,
Prevotella,
Escherichia coli,
Haemophilus influenza,
Rothia mucilanginosa,
Acinetobacter johnsonii,
Pseudomonas aeruginosa, and
Candida albicans, which promote the proinflammatory cascade of oral mucositis
[6].
Figure 2. Chemotherapy-induced modification of oral microbiota composition and homeostasis.
This entry is adapted from the peer-reviewed paper 10.3390/pathogens11040448