Water treatment generally does not specifically address the removal of microplastics (MPs). Nevertheless, treatment plants process water effectively, and the number of synthetic microparticles in effluents is usually very low. Still, discharge volumes from water-treatment plants are often elevated (reaching around 10^8 L/day), leading to the daily discharge of a substantial number of MPs and microfibers. Furthermore, MPs accumulate in the primary and secondary sludge, which in the end results in another environmental problem as they are currently used to amend soils, both for cultivation and forestry, leading to their dispersion. Something similar occurs with the treatment of water intended for human consumption, which has a much lower but still significant number of MPs.
- wastewater
- sewage sludge
- microplastics
- microfibers
1. Introduction
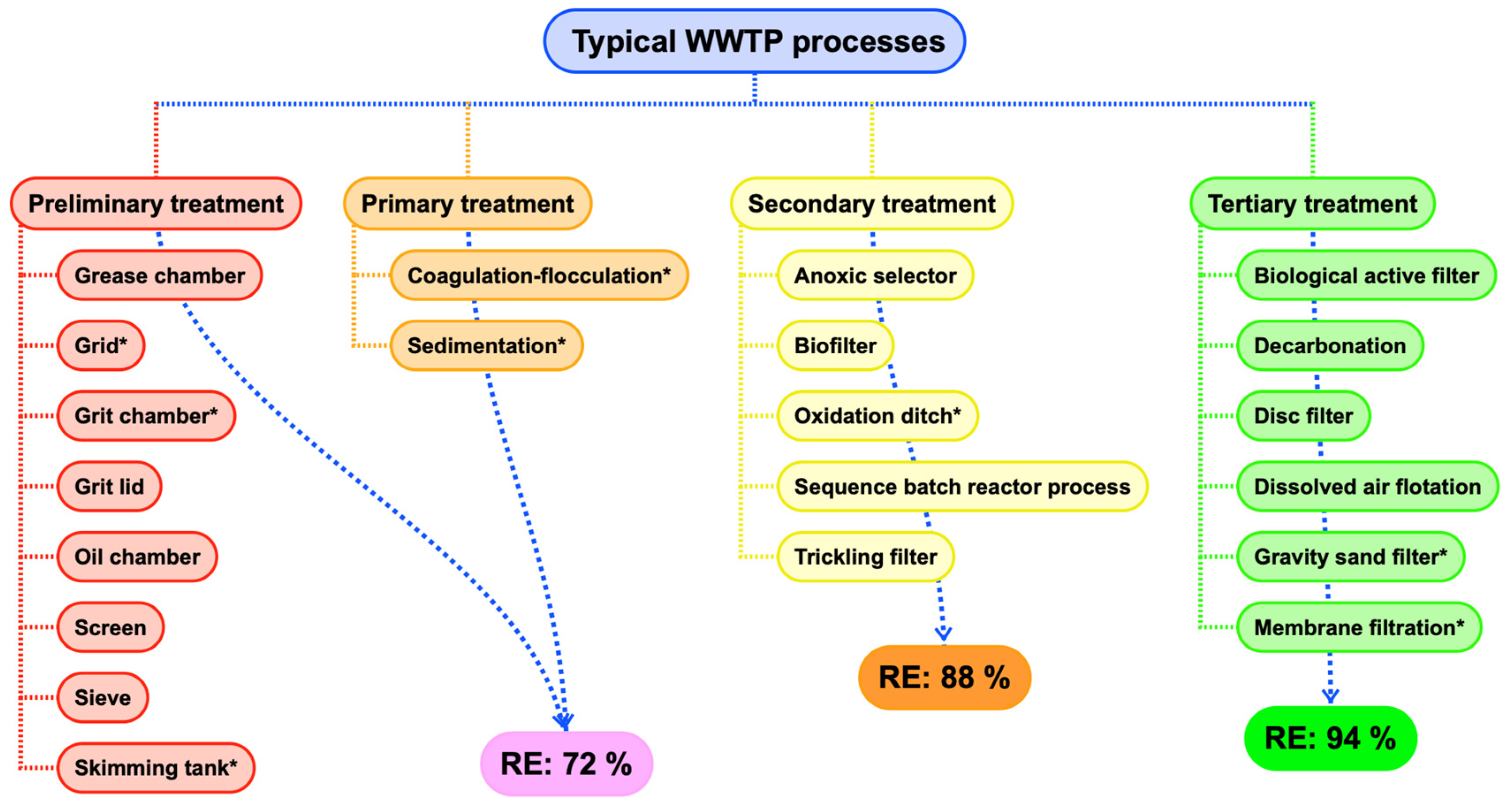
2. Microplastic Fate in Conventional Water/Wastewater-Treatment Processes
2.1. Microplastic Accumulation in Sludges
2.2. Need for Standardized Methods
This entry is adapted from the peer-reviewed paper 10.3390/en15072432
References
- UNEP Green Economy|UNEP-UN Environment Programme. Available online: https://www.unep.org/explore-topics/green-economy/what-we-do/economic-and-fiscal-policy/fiscal-policy/policy-analysis-4 (accessed on 16 February 2022).
- Pal, P. Selection of Water-Treatment Technology. In Industrial Water Treatment Process Technology; Elsevier: Amsterdam, The Netherlands, 2017; pp. 537–544.
- Cheng, Y.L.; Kim, J.-G.G.; Kim, H.-B.B.; Choi, J.H.; Fai Tsang, Y.; Baek, K. Occurrence and removal of microplastics in wastewater treatment plants and drinking water purification facilities: A review. Chem. Eng. J. 2021, 410, 128381.
- Iyare, P.U.; Ouki, S.K.; Bond, T. Microplastics removal in wastewater treatment plants: A critical review. Environ. Sci. Water Res. Technol. 2020, 6, 2664–2675.
- Spellman, F.R. Handbook of Water and Wastewater Treatment Plant Operations; CRC Press: Boca Raton, FL, USA, 2003.
- Zhang, X.; Chen, J.; Li, J. The removal of microplastics in the wastewater treatment process and their potential impact on anaerobic digestion due to pollutants association. Chemosphere 2020, 251, 126360.
- Cydzik-Kwiatkowska, A. Advanced wastewater treatment and biomass energy. Energies 2022, 15, 173.
- Zhang, Q.; Hu, J.; Lee, D.J.; Chang, Y.; Lee, Y.J. Sludge treatment: Current research trends. Bioresour. Technol. 2017, 243, 1159–1172.
- PlasticEurope-Association of Plastics Manufactures. Plastics—The Facts; European Association of Plastics Recycling and Recovery Organisations: Wemmel, Belgium, 2021.
- Iñiguez, M.E.; Conesa, J.A.; Fullana, A. Marine debris occurrence and treatment: A review. Renew. Sustain. Energy Rev. 2016, 64, 394–402.
- Conesa, J.A.; Iñiguez, M.E. Analysis of Microplastics in Food Samples. In Handbook of Microplastics in the Environment; Rocha-Santos, T., Costa, M., Mouneyrac, C., Eds.; Springer: Berlin/Heidelberg, Germany, 2020.
- Bayo, J.; López-Castellanos, J.; Olmos, S. Membrane bioreactor and rapid sand filtration for the removal of microplastics in an urban wastewater treatment plant. Mar. Pollut. Bull. 2020, 156, 111211.
- Prajapati, S.; Beal, M.; Maley, J.; Brinkmann, M. Qualitative and quantitative analysis of microplastics and microfiber contamination in effluents of the City of Saskatoon wastewater treatment plant. Environ. Sci. Pollut. Res. 2021, 28, 32545–32553.
- Hu, Y.; Gong, M.; Wang, J.; Bassi, A. Current research trends on microplastic pollution from wastewater systems: A critical review. Rev. Environ. Sci. Bio Technol. 2019, 18, 207–230.
- Cristaldi, A.; Fiore, M.; Zuccarello, P.; Conti, G.O.; Grasso, A.; Nicolosi, I.; Copat, C.; Ferrante, M.; Oliveri Conti, G.; Grasso, A.; et al. Efficiency of wastewater treatment plants (Wwtps) for microplastic removal: A systematic review. Int. J. Environ. Res. Public Health 2020, 17, 8014.
- De Falco, F.; Di Pace, E.; Cocca, M.; Avella, M. The contribution of washing processes of synthetic clothes to microplastic pollution. Sci. Rep. 2019, 9, 6633.
- Gies, E.A.; LeNoble, J.L.; Noël, M.; Etemadifar, A.; Bishay, F.; Hall, E.R.; Ross, P.S. Retention of microplastics in a major secondary wastewater treatment plant in Vancouver, Canada. Mar. Pollut. Bull. 2018, 133, 553–561.
- Bayo, J.; López-Castellanos, J.; Olmos, S. Abatement of Microplastics from Municipal Effluents by Two Different Wastewater Treatment Technologies. Water Pollut. XV 2020, 1, 15–26.
- Bayo, J.; Olmos, S.; López-Castellanos, J. Removal of Microplastics from Wastewater. In Handbook of Microplastics in the Environment; Rocha-Santos, T., Costa, M., Mouneyrac, C., Eds.; Springer: Berlin/Heidelberg, Germany, 2020.
- Murphy, F.; Ewins, C.; Carbonnier, F.; Quinn, B. Wastewater Treatment Works (WwTW) as a Source of Microplastics in the Aquatic Environment. Environ. Sci. Technol. 2016, 50, 5800–5808.
- Zhang, Z.; Chen, Y. Effects of microplastics on wastewater and sewage sludge treatment and their removal: A review. Chem. Eng. J. 2020, 382, 122955.
- Wu, M.; Tang, W.; Wu, S.; Liu, H.; Yang, C. Fate and effects of microplastics in wastewater treatment processes. Sci. Total Environ. 2021, 757, 143902.
- Johnson, A.C.; Ball, H.; Cross, R.; Horton, A.A.; Jürgens, M.D.; Read, D.S.; Vollertsen, J.; Svendsen, C. Identification and Quantification of Microplastics in Potable Water and Their Sources within Water Treatment Works in England and Wales. Environ. Sci. Technol. 2020, 54, 12326–12334.
- Liu, W.Y.; Zhang, J.L.; Liu, H.; Guo, X.N.; Zhang, X.Y.; Yao, X.L.; Cao, Z.G.; Zhang, T.T. A review of the removal of microplastics in global wastewater treatment plants: Characteristics and mechanisms. Environ. Int. 2021, 146, 106277.
- Rasmussen, L.A.; Iordachescu, L.; Tumlin, S.; Vollertsen, J. A complete mass balance for plastics in a wastewater treatment plant-Macroplastics contributes more than microplastics. Water Res. 2021, 201, 117307.
- Tadsuwan, K.; Babel, S. Microplastic contamination in a conventional wastewater treatment plant in Thailand. Waste Manag. Res. 2021, 39, 754–761.
- Bretas Alvim, C.; Bes-Piá, M.A.; Mendoza-Roca, J.A. Separation and identification of microplastics from primary and secondary effluents and activated sludge from wastewater treatment plants. Chem. Eng. J. 2020, 402, 126293.
- Simon, M.; van Alst, N.; Vollertsen, J. Quantification of microplastic mass and removal rates at wastewater treatment plants applying Focal Plane Array (FPA)-based Fourier Transform Infrared (FT-IR) imaging. Water Res. 2018, 142, 1–9.
- Hidayaturrahman, H.; Lee, T.-G.G. A study on characteristics of microplastic in wastewater of South Korea: Identification, quantification, and fate of microplastics during treatment process. Mar. Pollut. Bull. 2019, 146, 696–702.
- Ou, H.; Zeng, E.Y. Occurrence and Fate of Microplastics in Wastewater Treatment Plants; Elsevier Inc.: Amsterdam, The Netherlands, 2018; ISBN 9780128137475.
- Yang, L.; Li, K.; Cui, S.; Kang, Y.; An, L.; Lei, K. Removal of microplastics in municipal sewage from China’s largest water reclamation plant. Water Res. 2019, 155, 175–181.
- Talvitie, J.; Mikola, A.; Koistinen, A.; Setälä, O. Solutions to microplastic pollution—Removal of microplastics from wastewater effluent with advanced wastewater treatment technologies. Water Res. 2017, 123, 401–407.
- Lv, X.M.; Dong, Q.; Zuo, Z.Q.; Liu, Y.C.; Huang, X.; Wu, W.M. Microplastics in a municipal wastewater treatment plant: Fate, dynamic distribution, removal efficiencies, and control strategies. J. Clean. Prod. 2019, 225, 579–586.
- Talvitie, J.; Heinonen, M.; Pääkkönen, J.P.; Vahtera, E.; Mikola, A.; Setälä, O.; Vahala, R.; Paakkonen, J.P.; Vahtera, E.; Mikola, A.; et al. Do wastewater treatment plants act as a potential point source of microplastics? Preliminary study in the coastal Gulf of Finland, Baltic Sea. WATER Sci. Technol. 2015, 72, 1495–1504.
- Ben-David, E.A.; Habibi, M.; Haddad, E.; Hasanin, M.; Angel, D.L.; Booth, A.M.; Sabbah, I. Microplastic distributions in a domestic wastewater treatment plant: Removal efficiency, seasonal variation and influence of sampling technique. Sci. Total Environ. 2021, 752, 141880.
- Carr, S.A.; Liu, J.; Tesoro, A.G. Transport and fate of microplastic particles in wastewater treatment plants. Water Res. 2016, 91, 174–182.
- Liu, F.; Nord, N.B.; Bester, K.; Vollertsen, J. Microplastics Removal from Treated Wastewater by a Biofilter. Water 2020, 12, 1085.
- Ngo, P.L.; Pramanik, B.K.; Shah, K.; Roychand, R. Pathway, classification and removal efficiency of microplastics in wastewater treatment plants. Environ. Pollut. 2019, 255, 113326.
- Enfrin, M.; Dumée, L.F.; Lee, J. Nano/microplastics in water and wastewater treatment processes—Origin, impact and potential solutions. Water Res. 2019, 161, 621–638.
- Gatidou, G.; Arvaniti, O.S.; Stasinakis, A.S. Review on the occurrence and fate of microplastics in Sewage Treatment Plants. J. Hazard. Mater. 2019, 367, 504–512.
- Lares, M.; Ncibi, M.C.; Sillanpää, M.; Sillanpää, M. Occurrence, identification and removal of microplastic particles and fibers in conventional activated sludge process and advanced MBR technology. Water Res. 2018, 133, 236–246.
- Mahon, A.M.; O’Connell, B.; Healy, M.G.; O’Connor, I.; Officer, R.; Nash, R.; Morrison, L. Microplastics in sewage sludge: Effects of treatment. Environ. Sci. Technol. 2017, 51, 810–818.
- Habib, D.; Locke, D.C.; Cannone, L.J. Synthetic fibers as indicators of municipal sewage sludge, sludge products, and sewage treatment plant effluents. Water. Air. Soil Pollut. 1998, 103, 1–8.
- Jiang, J.; Wang, X.; Ren, H.; Cao, G.; Xie, G.; Xing, D.; Liu, B. Investigation and fate of microplastics in wastewater and sludge filter cake from a wastewater treatment plant in China. Sci. Total Environ. 2020, 746, 141378.
- Milojevic, N.; Cydzik-kwiatkowska, A. Agricultural use of sewage sludge as a threat of microplastic (Mp) spread in the environment and the role of governance. Energies 2021, 14, 6293.
- Wolff, S.; Weber, F.; Kerpen, J.; Winklhofer, M.; Engelhart, M.; Barkmann, L. Elimination of microplastics by downstream sand filters in wastewater treatment. Water 2021, 13, 33.
- Jellali, S.; Khiari, B.; Usman, M.; Hamdi, H.; Charabi, Y.; Jeguirim, M. Sludge-derived biochars: A review on the influence of synthesis conditions on pollutants removal efficiency from wastewaters. Renew. Sustain. Energy Rev. 2021, 144, 111068.
- Yan, P.; Shi, H.X.; Chen, Y.P.; Gao, X.; Fang, F.; Guo, J.S. Optimization of recovery and utilization pathway of chemical energy from wastewater pollutants by a net-zero energy wastewater treatment model. Renew. Sustain. Energy Rev. 2020, 133, 110160.
- Durdević, D.; Blecich, P.; Jurić, Ž. Energy Recovery from Sewage Sludge: The Case. Energies 2019, 12, 1927.
- The European Parliament; The Council of the European Union. European Parliament and of the Council Directive 2008/98/EC. Off. J. Eur. Union 2008, L 312, 3–30.
- Bannick, C.G.; Szewzyk, R.; Ricking, M.; Schniegler, S.; Obermaier, N.; Barthel, A.K.; Altmann, K.; Eisentraut, P.; Braun, U. Development and testing of a fractionated filtration for sampling of microplastics in water. Water Res. 2019, 149, 650–658.
- Bretas Alvim, C.; Mendoza-Roca, J.A.A.; Bes-Piá, A. Wastewater treatment plant as microplastics release source—Quantification and identification techniques. J. Environ. Manage. 2020, 255, 109739.
- Elkhatib, D.; Oyanedel-Craver, V. A Critical Review of Extraction and Identification Methods of Microplastics in Wastewater and Drinking Water. Environ. Sci. Technol. 2020, 54, 7037–7049.
- Ziajahromi, S.; Neale, P.A.; Rintoul, L.; Leusch, F.D.L. Wastewater treatment plants as a pathway for microplastics: Development of a new approach to sample wastewater-based microplastics. Water Res. 2017, 112, 93–99.
- Lusher, A.L.; Munno, K.; Hermabessiere, L.; Carr, S. Isolation and Extraction of Microplastics from Environmental Samples: An Evaluation of Practical Approaches and Recommendations for Further Harmonization. Appl. Spectrosc. 2020, 74, 1049–1065.
- Oßmann, B.E. Microplastics in drinking water? Present state of knowledge and open questions. Curr. Opin. Food Sci. 2021, 41, 44–51.
- Sun, J.; Dai, X.H.; Wang, Q.L.; van Loosdrecht, M.C.M.M.; Ni, B.J. Microplastics in wastewater treatment plants: Detection, occurrence and removal. Water Res. 2019, 152, 21–37.
- Hong, Y.; Oh, J.; Lee, I.; Fan, C.; Pan, S.Y.; Jang, M.; Park, Y.K.; Kim, H. Total-organic-carbon-based quantitative estimation of microplastics in sewage. Chem. Eng. J. 2021, 423, 130182.
- Lenz, R.; Labrenz, M. Small Microplastic Sampling in Water: Development of an Encapsulated Filtration Device. Water 2018, 10, 1055.
- Li, J.; Liu, H.; Paul Chen, J. Microplastics in freshwater systems: A review on occurrence, environmental effects, and methods for microplastics detection. Water Res. 2018, 137, 362–374.
- Dyachenko, A.; Mitchell, J.; Arsem, N. Extraction and identification of microplastic particles from secondary wastewater treatment plant (WWTP) effluent. Anal. Methods 2017, 9, 1412–1418.
