Geopolymer concrete (GPC) is a new material in the construction industry, with different chemical compositions and reactions involved in a binding material. The pozzolanic materials (industrial waste like fly ash, ground granulated blast furnace slag (GGBFS), and rice husk ash), which contain high silica and alumina, work as binding materials in the mix. The sustainable development can be achieved by employing geopolymers in construction industries, because it results in lower CO2 emissions, optimum utilization of natural resources, utilization of waste materials, low energy consumption, thermally stability, more cost-effective in long life infrastructure construction, and, socially, financial benefits and employment generation
- geopolymer concrete
- fly ash
- GGBFS
- compressive strength
1. Introduction
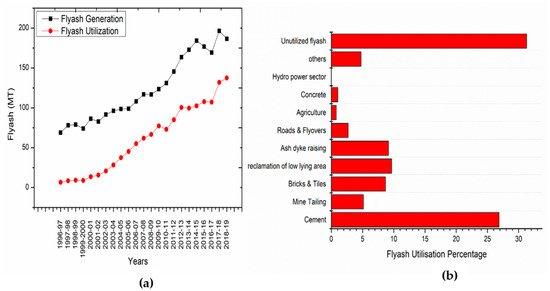
2. GPC Manufacturing Studies
2.1. Fly Ash Based GPC
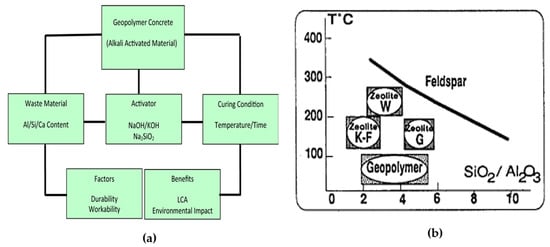
2.2. GGBFS Based GPC
2.3. Effect of Molar Ratios of Alkaline Solution
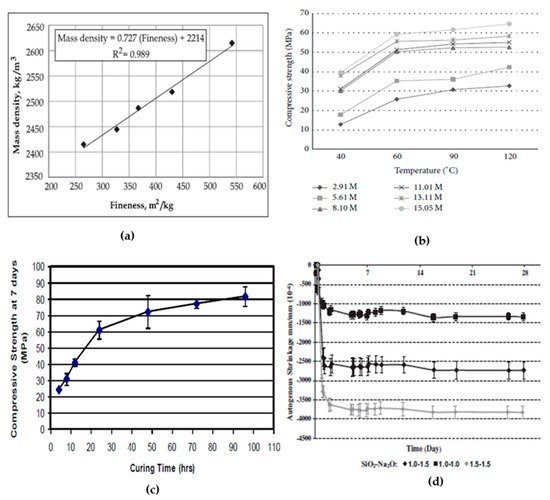
2.4. Effect of Calcination Temperature
2.5. Effect of Particle Size Fraction and Types of Aggregates
2.6. Effect of the Alkali Metal Activator
2.7. Effect of Ratio of Activator Liquid to Fly Ash/Slag
2.8. ITZ (Interfacial Transition Zone)
2.9. Effect of Curing Conditions
The calcium content in the slag and OPC is used in the GPC to form the CSH gel with the geopolymeric gel at low alkaline conditions and improve the mix’s compressive strength. The lower calcium content available to less CSH gel resulted in the mix’s lower overall strength. The calcium content plays a minor role in strength improvement in the high alkaline conditions and forms the CSH gels’ precipitation [81]. The high calcium BA’s mechanical strength depends on the fineness of the raw sample of BA and the water content in the mix design [82]. The calcium content present in the mix’s slag is essential for both early and more prolonged age. If the reaction rate is slow and low strength development confirmed, the low calcium fly ash is used as a binder with the lower concentration of alkali activator used without heat curing. The C-S-H/C-A-S-H precipitation formation initiated the strength development of the GPC fresh concrete, but in the fly ash GPC, the concrete hardens by forming alumina-silicate precipitation. The free calcium content from slag and fly ash dissolution increases the gel’s formation and later develops the hardened concrete’s strength [83]. The higher calcium in the mix emphasises the C-A-S-H type gel end product with a chain structure. A high amount of calcium content is attributed to high strength GPC [26,31].
Superplasticizer is used to increase concrete strength by lowering the water content of the mix design. The addition of a superplasticizer to the binder mass of up to 2% improves the workability of fresh GPC while having little effect on the strength of hardened GPC [30,84]. The use of superplasticizers is detrimental to the GPC's high-temperature performance [85]. The superplasticizer based on SNF is very effective for GPC [86]. The naphthalene-based superplasticizer is very useful, improving the workability of a slump by about 136%, while the PCE-based superplasticizer increases the workability by around 145%, but it affects the strength of the product blend specimens, reducing the strength by about 29%. In certain cases, SNF-based superplasticizer has no negative impact on concrete strength [87].
The addition of citric acid and sucrose to flyash-based GPC is an excellent alternative chemical admixture for improving the paste's rheological properties while increasing its weight. Sucrose acts as a retarder in the GPC mixture, while citric acid speeds up the hardening process. The mixture influenced the compressive strength after the sucrose was added, affecting the paste's porosity. Sucrose-added samples resulted in a relationship between compressive strength and porosity, directly attached to compressive strength. Sucrose could be used as a chemical admixture as a retarder in the GPC [88]. The polycarboxylate-based superplasticizer has a greater retarding effect on the flyash-slag-based GPC without affecting the paste's heat of hydration and has greater workability than the SNF-based superplasticizer. Increasing the content of PCE-based superplasticizer above 2% has little impact on the growing strength before 7 days, but it can have a detrimental effect on the strength of the GPC. The Superplasticizer improved the workability and mechanical strength of SCGC and the microstructure of bonding between the paste and aggregate at GPC's ITZ. The microstructure of ITZ differs due to changes in thickness caused by variations in superplasticizer material in the blend, which also influenced the compressive strength of GPC. By decreasing ITZ thickness, increased superplasticizer use improves the compressive strength of SCGC and the engineered quality microstructure. Superplasticizer material greater than 2% was inadequate to produce desirable workability with resistance to segregation.
In contrast, 6% and 7% of superplasticizer contents have the necessary workability properties within the EFNARC limits [89], with 7% producing the essential intensity at all ages and increasing the microstructural properties [71]. Because of its intrinsic resilience in alkaline media, The conventional superplasticizer is commonly used as an additive to the binder in OPC concrete to increase strength, but it degrades the hardened specimen strength in GPC blends. The use of a superplasticizer at high temperatures has a detrimental impact on the GPC mixed specimens. At higher temperatures, the SNF-based superplasticizer had no impact on strength. The high molar NaOH content in the GPC mix configuration makes the SNF-based helpful superplasticizer [5].
2.12. Effect of handling time
The fresh GPC is easily workable for up to 120 minutes without any strength deterioration [30]. The workability of the fresh GPC increases with increased hand mixing time up to 30 minutes [28]. If you increase the mixing time, it drastically retards the setting time of the fresh concrete.
2.13. Effect of silicate and alumina
In the SEM analysis, if the Si/Al ratio ≤ 1.40 is present in the matrix, it shows the clustered dense microstructure with large interconnected pores, and if Si/Al ≥ is 1.65, then the homogenous microstructure with tiny pores is present. The matrix gel's microstructure increases with the increase of the silicon content available when the ratio is 1.40 ≤ Si/Al ≤ 1.65. The geopolymer microstructure was affected by the absorption of nitrogen and resulted in the volume expansion of the matrix. The larger gel volume is responsible for higher compressive load and increases the young modulus when the microstructure of the gel is homogenous at the ratio of Si/Al is 1.65. So, the young modulus depends on both compressive strength and homogeneity in the microstructures of the gel. The mixed specimens' ultimate strength is reduced beyond the Si/Al = 1.90 due to the unreacted silica present in the matrix [90]. The thermal shrinkage increases as the mixed content's Si/Al ratio increases due to dehydration, dehydroxylation, sintering, and resilience [59]. The silica and alumina content play a vital role in the reaction of geopolymerisation. Silica content presents the amorphous end products in the reaction, contributing to the higher compressive strength of the mix design through the denser matrix development. The mechanical properties also increase with silica content and achieve a maximum strength of 65 MPa [91].
In the GPC, the SiO2/Al2O3 and SiO2/Fe2O3 ratios increase with the curing temperature, increasing the mechanical properties of the GPC. It also reduces the water absorption capacity compared to the OPC concrete. The CaO content present in the mix does not affect the geopolymer matrix's reaction [92]. The molar ratio of SiO2/Al2O3 increases to 3.4-3.8, which is highly responsible for the high strength gain at a later age [93]. In the flyash-based GPC, the Si: Al ratio ≥ 5 present in the flyash activated with sodium silicate shows low to moderate strength at ambient curing, but after the heat, the curing shows excellent dimensional stability and high compressive strength.
When the Si: Al ratio is less than 2, it shows high compressive strength but poor dimensional stability and reduces the strength after heating [94]. In the Geopolymerisation reaction, increases in alumina and silica content accelerate the range of 3.20–3.70. The mix alumina content increases neither show any zeolitic phase development nor shows the mix samples strength development [95]. The alumina content present in the mix is highly responsible for the setting time of the mix. Increasing the Si/Al ratio leads to a longer setting time, and also, increases in Al content decrease the strength of the concrete [94]. Figure 3d shows the effect of the SiO2/Na2O ratio on the autogenous shrinkage of the geopolymer paste.3. Durability and other related aspect studies
Geopolymers show a great potential in construction industry for durable structures subjected to extreme environment and for sustainable infrastructure development [96, 97]. In the durability studies, the long-term strength of the GPC deteriorates with time in aggressive environmental conditions [98]. Acid attack, seawater conditions, sulphate attack, carbonation of concrete, chloride penetration, alkali-aggregate reactions and free-thaw conditions were included in the durability studies.
3.1. Effect of Sulphate Attack
The magnesium sulphate deteriorates the GPC at a very high level in the calcium-rich geopolymer formed in the end products. It breaks the CSH bond and forms the Mg-SH by replacing the calcium present in the structure. The magnesium formed structure expanded the volume that created the crack formation in the GPC. At the same time, the sodium sulphate is not deteriorating the GPC at a very high level. The magnesium sulphate reduces the mechanical properties of the GPC mixed specimens [99]. The high-calcium BA Geopolymer mortar shows excellent resistant properties against sodium sulphate [83]. The Flyash/GGBFS-based GPC shows a 33% deterioration in mechanical strength and a 0.04% expansion after immersion in magnesium sulphate for 360 days, but the OPC concrete deteriorates to 48% mechanical strength and 0.8 expansion in concrete in the same conditions. In the Na2SO4, exposure to OPC concrete shows a deterioration of strength and expansion of 30% and 0.412%, respectively, but in the GPC, the strength is increased in the same condition [100]. The clay-flyash-based GPC is less affected by the sulphate attack on the GPC than the OPC concrete because the clay/flyash-based GPC contains significantly less calcium in the mix [101]. BFA-based GPC is significantly less susceptible to sulphate exposure after 18 months. The OPC concrete reduces up to 20%, but BFA-based GPC deteriorates up to 4% in strength in the same exposure condition as sodium sulphate [102]. Fig. 4a shows the expansion percentages of the various mixes in the exposure of MgSO4 and Na2SO4.
3.2. Effect of Acid Attack
The acid attack on the concrete decreases the concrete's performance and strength by reducing the specimens' mass loss in the acidic conditions below the 6.5 pH of the concrete. The sulphuric acid immersion in the exposure condition for 28 days shows the weakening of the concrete, and mass loss increased with the GPC matrix weakening. The loss reduction of concrete increases with the increase in acid content. The GPC shows better stability against acidic conditions than OPC concrete due to less calcium content present in the GPC [103]. In the slag-based geopolymer mortar, addition of nano-silica increase the micro-structure of cement paste (formation of additional calcium silicate hydrate (CSH) gels in the system) and strength properties [104]. If the pozzolanic content of geopolymer mortar is more than 50%, it shows better durable properties than conventional OPC concrete. It is less affected by acid attacks and chloride penetration in concrete. So, Alkali-Activated Slag/Fly Ash cement are highly useful in acidic or seawater conditions [105].
3.3. Effect of Sea Water
The GPC concrete shows better properties against seawater conditions by reducing the concrete's sulphate and chloride penetration [106]. The flyash-based GPC shows high compressive strength, tensile and flexural strength, low elasticity, water absorption, drying shrinkage, and sorptivity in seawater conditions. The flyash–based GPC achieved a strength of 55 MPa after 28 days, outperforming the OPC concrete, and was less susceptible to seawater in the same conditions [107].
3.4. Effect on Carbonation
The carbonation reaction rate of the GPC depends on the contents of the mixed design present in the concrete. The flyash/GGBFS based GPC shows weak resistance to the carbonation reaction due to the pozzolanic binder's activation by the mix's sodium silicate. The carbonation reaction increases the concrete's permeability, which is very hazardous for the concrete's durability [108].
3.5. Effect of Alkali-Silica Reaction and Leaching
The RCA dosage increases in conventional concrete reduce the concrete's strength and mechanical properties and lead to leaching in the concrete, but in the GPC, the RCA dosage does not affect the strength at a minimal level and reduces the leaching in the concrete [109]. The ASR susceptibility of flyash-based GPC is lower than that of OPC concrete [110,111]. The Nonwood biomass ash-based GPC shows excellent properties against acidic conditions compared to the OPC concrete because the OPC concrete shows 9% mass loss in 28 days under sulphuric acid conditions, while the biomass ash-based GPC shows less than 2% mass loss in the same conditions [112].
3.6. Effect of Elevated temperature
The aggregate size in the mix design of the concrete plays a vital role under high temperatures. If the maximum aggregate size is less than 10mm in the mix designs, it shows the explosive spalling of the concrete specimens under high temperatures in both types of concrete, GPC and OPC concrete. The spalling of concrete prevented using a maximum aggregate size of more than equal to 14mm in the concrete's design mix. The concrete's spalling is explained by the fracture process zone's size (lp), which varies with the aggregate size. The aggregate size is larger than the lp, also long and healthy because of the crack-tip shielding. The GPC is chemically stable under elevated temperatures, whereas the OPC concrete chemically decomposes and dehydrates under the same conditions and decreases in the evaporation water content, decreasing the spalling probability of the concrete [47]. The geopolymer mortars exhibited better performance at elevated temperatures in comparison to control cement mortar mixture [113]. The Si/Al ratio plays a vital role under the elevated temperature; the strength increases with the Si/Al ratio in the exposure of 800 0C of the mixed samples. The heat-cured specimens above 80 0C show higher stability against the elevated temperature. However, the ambient-cured specimens show lower stability in the same conditions, and potassium-based geopolymer shows higher stability than sodium-based geopolymer in high-temperature conditions [66,67]. The GPC has better stability against elevated temperatures than the OPC concrete and is more porous than the OPC concrete analysed by the sorptivity test. It reduces the risk of the spalling of concrete under high-temperature conditions [114].
The FSGC shows similar trends to that of Portland cement concrete in weight loss under elevated temperatures of 600 0C [115]. The GPC shows a higher degree of transient creep and the OPC pastes below the 250 0C temperature. When the temperature ranges between 250 0C and 550 0C, the geopolymer does not show the transient creep increase while the OPC paste shows the higher transient creep, and the geopolymer increases the elastic modulus. OPC concrete shows a minor change in the elastic modulus [116]. The powder slag of ferrosilicon (FSS), an industrial waste, may be blended for manufacturing light weight GPC to conserve its strength properties under elevated temperature [117]. The electric arc furnace steel slag (EAFSS), barite and ilmenite heavy aggregates based heavyweight geopolymer concrete is very effective in radiation shielding at high temperatures [118]. The dosage of the MPCM shows the stimulating effects on the thermal performance of the PCC and GPC. The number of microcapsules affects the thermal conductivity and latent heat of concrete. Microcapsule dosage increases concrete porosity and has more substantial effects on GPC than PCC [119]. The geopolymer matrix's thermal conductivity is higher than the OPC pastes and shows that the geopolymer paste's specific heat is less than the PC pastes [120]. The pore size distribution plays a vital role in the FA/M-based GPC under elevated temperatures [121].
3.7. Effect on the Bond Strength
The GPC specimens explain a similar cracking pattern to the OPC concrete in the pull-out load test, and both fail in a brittle manner due to the splitting of concrete along with the bonding of concrete and bars. The bond strength increases with the concrete strength and concrete cover in both types of concrete. GPC shows higher bond strength than OPC concrete due to the higher splitting strength of GPC for the same compressive strength[122,123]. The GPC beams with lap spliced reinforcement show similar failure behaviour to the OPC concrete beams. For both types of concrete, the reinforcement is based on Australian Standards and the ACI code. The bond strengths of the beam-ends specimens have lower strength compared to the direct pull-out tests.The bar size plays a vital role in the bond strength; the bond strength increases with the reduction of the bar size[124]. The bond strength of the sand-coated GFRP-reinforced GPC compared to OPC concrete shows higher failure loads than the OPC concrete [125].
The ultimate strength and crack load increase with the increment of the fibre concentration in the mix designs, and it also reduces the cracking rate in the beam [126]. The sand-coated GFPR bars are a perfect alternative to the internal reinforcement of GPC structures [127]. The elastic behaviour of the GPC under reinforced beams is similar to the under-reinforced OPC concrete beams. GPC specimens show a more brittle flexural strength than OPC concrete specimens [122,128]. The compressive strength of the GPC increased by 5% with the 1% dosage of the 1% steel fibre in the mix [129]. The load capacity of fly ash/GLSS-based geopolymer concrete column increases with the increment of concrete strength, reduction in the load eccentricity, and increasing the design's longitudinal-reinforcement ratio [130]. The column-designed specimens show a similar failure to the design code AS3600 [131].4. Geopolymer material applications
The GPC materials have found number of application in infrastructure development and different fields [132]. The state-of art for the potential applications of geopolymers in the sustainable construction are given in [133, 134]. Researches are conducted for barriers and challenges in the effective application of GPC and reinforced geoploymer composites [135,136]. The geopolymers are successfully used for soil stabilization in transportation and geotechnical engineering [137, 138]. The geopolymer are most suitable for marine engineering construction due to their corrosive resistance and excellent aggressive environment performances [139]. The geopolymers can be used to construct light weight [140] and blast resistant structure [141]. The geopolymers are used in buildings for floor heating [142] and for energy saving applications i.e. to reduce HVAC demand, of building [143]. GEO-based materials can be a sound choice in construction industries in place of OPC for sustainable development due to their thermal energy storage capacity [144], fire retardant capabilities [145], and electrical and self-sensing characteristics [146]. The geopolymer is cost-effective due to its stable performance against elevated temperatures and is used to replace epoxy resins in structural retrofitting with FRP. It is also used as a cost-efficient lining of the trenches to rehabilitate sewage pipelines [147,148]. The geopolymers prove as sound material for structural retrofitting and rehabilitation of heritage buildings [149, 150], for building preservation [151, 152], for damage structural elements [153].
Geopolymer as aggregates in concrete shows better strength and stability compared to natural aggregates [154]. The geopolymers meet the desired requirements as a material for 3D printing construction [155, 156]. The 3D printed GPC can be potentially used in a wide range of structural applications in construction industry due to its sustainable processing [157, 158].|
Si/Al ratio |
Applications |
|
1 |
Ø Bricks Ø Ceramics |
|
2 |
Ø Low CO2 Cements and Concretes Ø Radioactive and toxic waste encapsulation |
|
3 |
Ø Foundry equipment Ø Thermal insulation materials, 200-10000C Ø Tooling for titanium Processing |
|
>3 |
Ø Sealant for industry, 200-6000C Ø Tooling for aeronautics SPF aluminium |
|
20-35 |
Ø Fire-resistant and heat resistant fibre composites |
5. Sustainability
The composition of review for sustainability of geopolymer concrete is due to [160, 161]. In terms of energy used in the production of concrete constituents, sustainability was increased by using alternative materials and reducing energy consumption [162]. In the production of GPC, industrial solid waste is used as a binder, activated by alkaline chemicals for concrete production [163]. In the PCC, cement, aggregate (fine and coarse), and water are used to produce it. The GPC production uses flyash, GGBFS, NaOH, Sodium Silicate, aggregate (fine and coarse), and water. In India, cement production's mostly dry process generates the energy of 4.2 MJ/kg [164]. The flyash and GGBFS are solid industrial wastes produced by the thermal power plant and steel plant. The flyash is directly used in the mix, but the GGBFS requires grinding before use in the mix. NaOH production's embodied energy is 20.5 MJ/kg, and sodium silicate production is 5.37 MJ/kg [165]. There is zero embodied energy in flyash and water. The coarse aggregate and fine aggregates' embodied energy are 0.22 MJ/kg and 0.02 MJ/kg, respectively. The superplasticizer's embodied energy in SNF-based is 12.6 MJ/kg. The total embodied energy of the OPC concrete is 1897.86 MJ/m3, whereas the embodied energy of the GPC is 1749.21 MJ/m3. The GPC’s embodied energy is less compared to the OPC concrete. Table 2 describes the total constituents present in both the mix design and their embodied energy.
Table 2. GPC and OPC concrete constituents details
|
|
Embodied Energy (MJ/kg) |
OPC Concrete |
Geopolymer Concrete |
||
|
Mix Content (kg/m3) |
Embodied Energy Content (MJ/kg) |
Mix Content (kg/m3) |
Embodied Energy Content (MJ/kg) |
||
|
OPC |
4.2 |
370 |
1554 |
00 |
00 |
|
Flyash |
0.0 |
00 |
0.0 |
303.75 |
00 |
|
GGBFS |
0.31 |
00 |
00 |
101.25 |
31.38 |
|
NaOH |
20.5 |
00 |
00 |
40.5 |
830.25 |
|
Na2SiO3 |
5.37 |
00 |
00 |
101.25 |
543.71 |
|
Fine Aggregate |
0.02 |
683 |
13.66 |
683 |
13.66 |
|
Coarse Aggregate |
0.22 |
1289 |
283.58 |
1269 |
279.18 |
|
Water |
0.0 |
148 |
00 |
40.5 |
00 |
|
Superplasticizer |
12.6 |
3.7 |
46.62 |
4.05 |
51.03 |
|
|
Total |
2493.7 |
1897.86 |
2543.7 |
1749.21 |
The geopolymers materials promotes circular economy process and sustainable development [166, 167]. The sustainable material, such as geopolymer, application and economical production techniques in the construction industry create employment and increase energy efficiency [168]. The economic and commercial manufacturing aspects (social), an important component of sustainability, of the GPC has not been paid much attention. The cost studies of GPC are due to Mathew et al. [169]. The GPC is more cost effective than GPC. Weil et al. [170] have studied the cost comparison and cost drivers for OPC concrete and GPC. The cement is the main cost driver in cement concrete while the activators are the cost driver in GPC. The cost-effectiveness of GPC manufacturing is demonstrated by Youssef et al. [171]. van Deventer et al. [172] has discussed the factors affecting the acceptance of GPC technology in industry. They pointed out that the regulations and supply chain issues barrier are to be removed for wide spread acceptance of GPC. Shamsaei et al. [173] have reviewed the studies devoted to the obstacles related to commercialization of GPC. They pointed out that economic analysis and social/national standards attitude towards the application of GPC requires further research. The 3D printed geopolymer concrete is cheaper as compared to 3D-printed cement concrete with the same properties [174]. It can be con[1][2][3][4][5][6][7][8][9][10][11][12][13][14][15][16][17][18][19][20][21][22][23][24][25][26][27][28][29][30][31][32][33][25][34][35][36][37][38][39][40][25][41][42][43][44][45][46][47][48][49][50][51][52][53][54][55][56][57][58][59][60][61][62][63][64][65][66][67][68][69][70][71][72][73][74][75][76][77][78][79][80][81][82][83][84][85][86][87][88][89][90][91][92][93][94][95][96][97][98][99][100][101][102][103][104][105][106][107][108][109][110][111][112][113][114][115][116][117][118][119][120][121][122][123][124][125][126][127][128][129][130][131][132][133][134][135][136][137][138][139][140][141][142][143][144][145][146][147][148][149][150][151][152][153][154][155][156][157][158][159][160][161][162][163][164][165][166][167][168][169][170][171][172][173][174]cluded that the sustainable development is achieved by employing geopolymers in Indian construction industries because it results in lower CO2 emission, optimum utilization of natural resources, utilization of waste materials, economic and long life infrastructure construction and society income and employment generation.
This entry is adapted from the peer-reviewed paper 10.3390/cryst12040514
