1. Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is an enveloped (+)ssRNA virus, in which the infecting RNA acts as a messenger RNA (mRNA). After entering the host cell, SARS-CoV-2 is replicated. This process involves the translation of viral mRNA by cellular ribosomes to produce the viral replicative enzymes, which generate new RNA genomes and the mRNAs for the synthesis of the components necessary to assemble the new viral particles [
1].
SARS-CoV-2 is the cause of a global pandemic of coronavirus disease of 2019 (COVID-19). On 31 December 2019, the World Health Organization’s (WHO) country office in China registered cases of ‘viral pneumonia’ in Wuhan. A month later, on 30 January 2020, WHO’s Director-General declared the novel coronavirus outbreak a public health emergency of international concern. On 11 March 2020, WHO made the assessment that COVID-19 could be characterized as a pandemic. Globally, as of 4 April 2022, there have been 494 million confirmed cases of COVID-19, including 6.15 million deaths. The highest number of COVID-19 cases, some 80.1 million, were in the United States, and included 0.98 million deaths. The scale of health and economical threats caused by the pandemic outbreak urged many scientific groups to research the mechanisms triggered by the virus to allow treatment and vaccination. Several SARS-CoV-2 variants have emerged since the first identified strain, apparently with higher transmissibility/virulence and immune escape capabilities.
Interestingly, COVID-19 patients present a diverse severity of clinical manifestations, ranging from no symptoms to death. Of the total COVID-19 cases, about 80% are either asymptomatic or experience a mild course of the disease, while about 14% develop severe symptoms, such as pneumonia, and about 5% present critical symptoms, such as septic shock, respiratory failure, or multi organ failure, and finally about 2% of the subjects die. In general, the worse course of the disease is associated with old age and comorbidities, especially chronic obstructive lung disease, obesity, diabetes mellitus, cardiovascular disease and hypertension [
2].
To complicate matters, in a considerable fraction of patients, SARS-CoV-2 infection is followed by a complication called long COVID-19, which can last for months and has diverse symptoms such as fatigue, headache, ‘brain fog’, anosmia, myalgia, dizziness, breathlessness, palpitations, and gastrointestinal problems. The prevalence of long COVID-19 is based on ten reporting studies, and ranged from 4.7% to 80%. The frequency of most prevalent long COVID-19 symptoms that may last from weeks to months after acute infection was as follows: chest pain—up to 89%, fatigue—up to 65%, dyspnea—up to 61%, cough and sputum production—up to 59%, cognitive and memory impairment—up to 57.1%, arthralgia—up to 54.7%, sleep disorders—up to 53%, and myalgia—up to 50.6%. The list of other signs and symptoms of long COVID-19 with lower frequency contains over thirty records, all of which are listed with reporting studies [
3].
The progression of COVID-19 can be divided into three overlapping phases: early infection, pulmonary phase and hyperinflammation [
4]. As the lung parenchyma is targeted by the virus, the organism activates innate immune response, and the following effects may be triggered: inflammation, damage to the vessel walls, vasodilation and endothelial permeability, pulmonary restriction, hypoxemia, and increased cardiovascular stress. Respiratory failure, if present, together with viral infiltration into myocardial tissue and cardiac inflammation leads to cardiac injury [
4]. Kumar et al. [
2] have described the COVID-19 mechanisms in the human body, including symptomatology, virus–host interactions, and host factors affecting transmissibility, severity and outcomes (age, sex and comorbidities) as well as organ-specific pathologies ongoing in the respiratory, cardiovascular, renal, digestive, and nervous systems during SARS-CoV-2 infection [
2].
A summary of over twenty proteomic studies on plasma and serum of COVID-19 patients revealed three deregulated KEGG pathways: complement and coagulation cascades, cytokine-cytokine receptor interaction and cholesterol metabolism [
5]. Elevations of inflammation biomarkers such as IL (interleukin)-6, IL-2, IL-7, TNF (tumor necrosis factor), MCP (monocyte chemoattractant protein)-1, MIP (macrophage inflammatory protein)-1, G-CSF (granulocyte-colony stimulating factor), CRP (C-reactive protein), procalcitonin and ferritin are associated with increased mortality [
4] and higher disease severity [
6]. The results of a cohort study with 84 patients diagnosed with COVID-19 from Wuhan, China, demonstrated that the level of cardiac enzymes, as well as the abnormalities in the ECG, correlate positively with the level of inflammation values, in particular CRP and procalcitonin [
7]. As shown recently, multi organ failure in patients with severe COVID-19 complication is caused by systemic vasculitis and cytokine mediated coagulation. Other identified biomarkers are hematological (lymphocyte count, neutrophil count, neutrophil-lymphocyte ratio), erythrocyte sedimentation rate, D-dimer, troponin, creatine kinase, and aspartate aminotransferase. Homocysteine (Hcy) and angiotensin II were also suggested to play significant roles [
8].
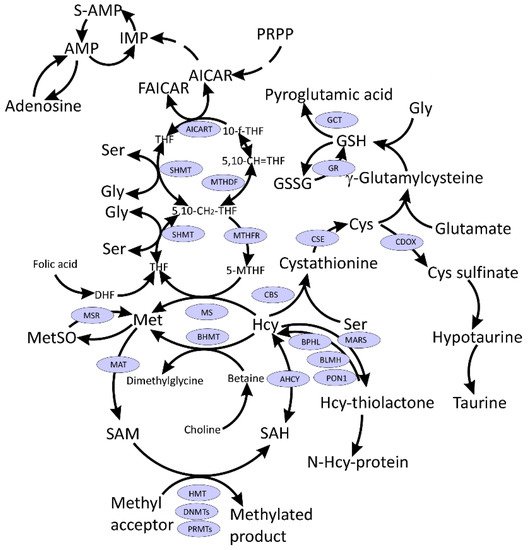
The symptoms of long COVID-19 are similar to those presented by subjects suffering from pernicious anemia (a condition caused by vitamin B
12 deficiency), where methylation status is compromised [
9,
10]. Vitamin B
12 is a cofactor of the key one-carbon metabolism enzyme—vitamin B
12-dependent methionine (Met) synthase (MS) that remethylates Hcy to Met and links Met and folate cycles (
Figure 1). MS generates Met, which then is used for the production of S-adenosylmethionine (SAM), a universal methyl donor, for a variety of acceptors, many of which participate in epigenetic regulation of gene expression. Moreover, one-carbon metabolism supports multiple physiological processes, such as biosynthesis of purines and thymidine, amino acid homeostasis of glycine (Gly), serine, and Met, and underlies antioxidant defense via glutathione (GSH) (
Figure 1). Additionally, one-carbon metabolism is also important in the generation of energy via adenosine triphosphate (ATP) production in the mitochondria [
9,
10,
11].
Figure 1. Interconnections between metabolism of folate, one-carbon, and sulfur compounds. Indicated metabolites are discussed in the text. CysGly, a product of GSH catabolism, affected in COVID-19 and discussed in the text, is not shown.
Accumulating evidence suggests that one-carbon metabolism plays an important role in COVID-19. The purpose of this review is to summarize recent findings related to sulfur amino acids and one-carbon metabolism in COVID-19 and discuss how they inform strategies to combat the disease.
2. SARS-CoV-2 Hijacks Host Folate and One-Carbon Metabolism
Metabolism of a cell infected by a virus is reshaped to fulfill the viral needs for its successful replication. Under viral infection, anabolic reactions are dominant and there is an upregulation of the ingestion of an extracellular carbon source (e.g., glucose or glutamine), which is used for lipogenesis and nucleotide synthesis, both of which are crucial for viral replication [
1].
Several amino acids and their derivatives play important roles in one-carbon metabolism (Figure 1). Activated Met in the form of SAM is a universal methyl donor, Hcy is an intermediate in Cys synthesis, which in turn is needed for glutathione synthesis responsible for redox homeostasis. Ser is the precursor for the biosynthesis of several amino acids including Gly and Cys and participates in folate metabolism by donating one-carbon units for the biosynthesis of purines and pyrimidines.
Insight into how SARS-CoV-2 remodels the host metabolism to support the virus replication has been provided by ex vivo studies using the African Green Monkey Vero E6 cells [
12]. Upon infection with SARS-CoV-2 isolate, the cells rapidly produced viral genomic RNA and nucleocapsid protein by 8 h post-infection, at which time the induction of antiviral genes, NF-ĸB targets, and ER stress response was observed. These changes were accompanied by a global decrease in host mRNAs with no changes in levels of mRNAs encoding metabolic enzymes. The changes in the patterns of mitochondrial DNA expression indicated ATP depletion. One of the most striking changes was the elevation of
de novo purine synthesis intermediates (PPRP, FGAR, AIR, SAICAR) (
Figure 1) in the SARS-CoV-2-infected cells and the reduction of intracellular folate levels (
Table 1). Other metabolites participating in one-carbon metabolism and sulfur amino acid metabolism were also affected by the SARS-CoV-2 infection (Supplementary Data 4 in Ref. [
12]). For example, intracellular Met, cystathionine, pyridoxine, betaine, serine, Gly, 5-oxoproline (pyroglutamate), and cysteine-glutathione disulfide levels were attenuated, while reduced glutathione levels were elevated. Intracellular SAM, SAH, cysteine (Cys), oxidized glutathione (GSSG) levels were not affected in the SARS-CoV-2-infected cells. These findings suggest that SARS-CoV-2 hijacks folate and one-carbon metabolism to meet the demands for viral replication [
12]. As shown in
Table S1 and discussed below, these metabolites were also affected by SARS-CoV-2 infection in vivo in COVID-19 patients.
Table 1. Intracellular metabolites affected by the SARS-CoV-2 infection of Vero E6 cells.
| Metabolite |
Fold Change 1 |
p Value 2 |
| Purine biosynthesis |
| Folate |
0.62 |
0.0020 |
| 5-Formimino-tetrahydrofolate |
0.18 |
0.0018 |
| Serine |
0.87 |
0.0029 |
| Glycine |
0.71 |
0.0025 |
| Ribose-5-Phosphate/Xylulose-5-phosphate |
0.91 |
0.405 |
| 5-Phosphoribos-1-pyrophosphate (PRPP) |
1.44 |
0.005 |
| Formylglycinamide ribonucleotide (FGAR) |
2.38 |
2 × 10−6 |
| Aminoimidazole ribonucleotide (AIR) |
3.10 |
0.0113 |
| Succinylaminoimidazolecarboxyamide (SAICAR) |
1.24 |
0.0218 |
| Methionine cycle |
| Methionine |
0.68 |
0.0020 |
| S-Adenosylmethionine (SAM) |
1.01 |
NS |
| S-Adenosylhomocysteine (SAH) |
1.19 |
NS |
| Trans-sulfuration pathway |
| Cystathionine |
0.70 |
0.0507 |
| Cysteine |
0.80 |
NS |
| Glutathione biosynthesis |
| Pyroglutamate/5-Oxoproline |
0.73 |
0.0028 |
| Glutathione, reduced (GSH) |
1.71 |
0.0012 |
| Glutathione, oxidized (GSSG) |
0.97 |
NS |
| Cysteine-glutathione disulfide |
0.34 |
0.0025 |
| Taurine biosynthesis |
| Cysteinesulfinic acid |
3.10 |
0.0291 |
| Choline |
1.33 |
0.0014 |
| Betaine |
0.78 |
0.0497 |
3. S-Adenosylmethionine and Methylation Index
The SAM/SAH ratio, known as the methylation index, may be affected by SARS-CoV-2 infection. As mentioned earlier, SAM is required for capping of the viral RNA. The RNA cap (m7GpppN-RNA) is composed of a 7-methylguanosine (m7G) linked to the 5′-nucleoside (N) of the RNA chain through a triphosphate bridge (ppp). The cap structure is methylated at the N7 position of the guanosine by the C-terminal (guanine-N7)-methyltransferase (N7-MTase) domain of nonstructural protein 14 (Nsp14), forming cap-0 (m7GpppN-RNA), using SAM as a methyl donor [
13,
14]. The second methylation reaction during cap synthesis is catalyzed by SAM-dependent Nsp16 methyltransferase, which adds the methyl group on the ribose 2′-O position of the first transcribed nucleotide to form cap-1 (m7GpppNm-RNA). The RNA final cap has several important biological roles in viruses as it is critical for the stability of mRNAs, both for their translation and to evade the host immune response [
14].
It has been hypothesized that SARS-CoV-2 infection may lead to SAM depletion in patients suffering long-term consequences of COVID-19. However, although SAM has not been quantified in long COVID-19, this hypothesis doesn’t seem to hold much water because several studies showed significant increases or no changes in plasma SAM levels in COVID-19 cases (
Table S1). Moreover, SAH levels are either elevated [
15,
16], attenuated [
17] or do not change in COVID-19 [
18]. For example, a study on fifty-six COVID-19 patients admitted to the hospital between September and December 2020 in Moscow, Russia, has shown that an elevated SAM level and SAM/SAH and SAM/glutathione ratios have been associated with an increased risk of severe lung injury. Furthermore, an elevated SAM concentration and SAM/SAH and SAM/GSF ratios have been associated with an increased risk of lung damage [
18]. Metabolomic analyses have revealed that SAM was significantly elevated in critical cases of COVID-19 [
15] and those with a fatal outcome [
19] as compared to control, mild and moderate cases of COVID-19 [
15] (
Table S1). Even though SAM was highest among severe COVID-19 patients, it was associated with a favorable prognosis. On the other hand, while there was no association between dimethylglycine, a by-product of Hcy remethylation to Met by a betaine-dependent enzyme BHMT (
Figure 1), and COVID-19 stage, dimethylglycine was significantly lower in patients with an unfavorable progression of COVID-19 [
15].
4. Methionine and Methionine Sulfoxide
The results of metabolomic studies on Met are contradictory, showing upregulation [
16], downregulation [
20,
21], or no change in Met levels [
17,
22] in COVID-19 cases vs. healthy controls (
Table S1). The direction of changes in Met level depends on compared groups, i.e., there is a tendency to higher Met levels in critical COVID-19 patients vs. healthy controls, but Met was lower in mild COVID-19 patients vs. healthy controls and there was no change in Met levels in patients with moderate COVID-19 vs. controls [
15].
Metabolomic analyses of blood samples from COVID-19 patients and COVID-19-negative subjects revealed the significant impact of SARS-CoV-2 infection on serum Met sulfoxide, which consistently showed increased levels in four independent studies, suggesting increased oxidant stress [
16,
17,
23,
24] (
Table S1).
5. Glutathione and Related Metabolites
COVID-19 is associated with disrupted redox homeostasis and reactive oxygen species (ROS) accumulation. In May 2020, Polonikov published a hypothesis which stated that [
25]: “glutathione deficiency is the most plausible explanation for serious manifestation and death in COVID-19 patients”. Glutathione (GSH) depletion has been observed in diseases that increase the risk of COVID-19 [
26]. GSH, being the main antioxidant agent, was suggested to be essential for counterbalancing the inflammation observed in SARS-CoV-2 infected patients (reviewed in Ref. [
27]).
The glutathione hypothesis of COVID-19 appears to be supported by available data (
Table S1). Indeed, GSH levels are consistently decreased in COVID-19 patients [
17,
18,
28,
29]. For example, a study on fifty-nine COVID-19 patients admitted to the hospital between August and November 2020 in Moscow, Russia [
29], found that the levels of total GSH (tGSH) were significantly lower in moderate and severe COVID-19 patients compared with mildly affected subjects, while reduced CysGly (rCG) was significantly decreased in patients with higher degrees of lung damage based on percentage of lobar involvement (>26%) as compared to subjects with a lower degree of lung damage (0–25% of lobar involvement) (
Table 2). tGSH and rCG were suggested to be risk markers for the severity of COVID-19 and lung damage in patients [
29]. In addition, a negative correlation between rGSH and advance oxidation protein products in patients with high lung damage was observed [
29]. A similar study involving fifty-six COVID-19 patients admitted to the hospital between September and December 2020 in Moscow, Russia [
18], found lower GSH concentration in patients with a higher degree of lung damage (>50% of lobar involvement) as compared to patients with a lower degree of lung injury (<25% of lobar involvement). There also has been a significant increase in SAM level and SAM/GSH ratios, and a tendency to higher Hcy levels in subjects with more injured lungs [
18] (
Table 2).
Table 2. Sulphur metabolites in COVID-19 patients stratified by a degree of lung damage.
| Metabolite |
Degree of Lung Damage |
References |
| CT0–1, <5–25% (n = 26) |
CT2, 26–49%
(n = 16–18) |
CT3–4, 50–75% (n = 14–15) |
|
| tGSH, µM |
1.81 |
1.15 |
1.22 * |
[29] |
| rCG, µM |
1.59 |
1.30 |
1.29 * |
| GSH, µM |
1.81 |
1.15 |
1.22 # |
[18,29] |
| Hcy, µM |
7.4 |
8.3 |
9.1 |
[18] |
| SAM, nM |
59 |
57 |
84 # |
| SAM/GSH, nM/µM |
3.6 |
7.2 & |
5.5 |
| SAM/GSH, nM/µM |
32 |
57 |
60 # |
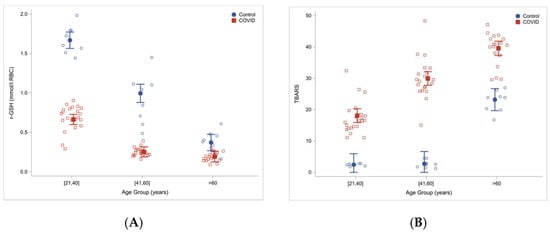
Another study involving sixty COVID-19 patients hospitalized in Houston, TX and twenty-four uninfected controls, found that total and reduced red blood cells GSH were significantly lower in COVID-19 patients then in controls. At the same time, measures of lipid peroxidation, indicating oxidative stress (TBARS and F2-isoprostanes), were significantly elevated in COVID-19 patients and increased with age [
28]. Severe GSH deficiency and oxidative damage also occur in young COVID-19 patients, and the magnitude of these defects in COVID-19 increased with age (
Figure 2).
Figure 2. Reduced glutathione concentrations (
A) and increased oxidative stress (TBARS) (
B) in COVID-19 patients and uninfected controls stratified by age. Reproduced from Ref. [
28] with permission.
Cys levels were elevated in plasma [
24], decreased in serum [
21] and plasma [
20] or unchanged in serum [
22] and plasma [
15]. Cystine, the oxidized disulfide form of Cys, was elevated in serum and plasma in several studies [
16,
21,
22] and downregulated with IL-6 increase [
23]. In another study, Cys was elevated in moderate COVID-19 patients vs. controls; however, analysis of critical and mild COVID-19 patients showed no changes in Cys levels vs. control group [
15]. Cystathionine, an intermediate in the transsulfuration pathway (
Figure 1), was either upregulated in plasma [
30] and serum [
17] or downregulated in the plasma of COVID-19 patients [
24]. CysGly, a product of GSH metabolism, was shown to decrease in patients with higher degree of lung damage as diagnosed by computer tomography [
29] or increase in plasma of COVID-19 patients as compared with controls [
16].
Gly, which participates in GSH biosynthesis, was found to either increase [
22], decrease [
15,
23], or was unchanged [
16,
17,
21,
31] in COVID-19 patients. In addition, pyroglutamate, a metabolite that forms from γ-glutamyl-Cys (glutathione precursor) (
Figure 1) when Gly is limiting, was shown to be downregulated in COVID-19 patients [
17] (
Table S1).