Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
C6F12O is in great demand on the market with its excellent environmental performance and broad application prospects. It is of great significance to the development of a synthetic route for C6F12O, suitable for industrial production.
- C6F12O
- Novec 1230
- Synthesis Route
1. Introduction
Chemical gas fire suppressants are widely applied in fire protection systems due to their high fire extinguishing efficiency, stable, clean, convenient storage and transportation characteristics, and their easy installation and maintenance [1][2]. Among them, ozone-depleting substances (ODSs) [2] containing chlorine or bromine such as CF3Br (Halon 1301), a kind of bromofluorocarbons (halon), once widely applied, have been phased out under the Montreal Protocol (1987), except for some specific applications in aircrafts and ships [3][4][5]. The search for proper fire suppressants with ozone depletion potential (ODP) close to 0 has become one of the key indicators for halon replacements.
Gas fire suppressants substituted for halon are mainly divided into inert gases (IGs) and hydrofluorocarbons (HFCs). IGs, such as CO2, N2, Ar and their mixtures, with their large storage space, low safety margin and poor fire extinguishing efficiency, have limited applications. HFCs including heptafluoropropane (HFC 227ea, FM200), pentafluoroethane (HFC 125, FE27), hexafluoropropane (HFC 236fa, FE36), trifluoromethane (HFC 23, FE13), etc., with no Cl, Br atoms (ODP is 0), and a fire extinguishing performance close to halon are widely used as halon substitutes at present. However, HFCs belong to greenhouse gases [3] and the global warming potential (GWP) is extremely high. Meanwhile, HFCs cannot completely replace halon in aircrafts, ships and some other fields [4], so these substances can only be treated as transitional fire suppressants in the process of halon replacement. The Kyoto Protocol (1997) and the Kigali Amendment to the Montreal Protocol (2016) have set a deadline for the phasing out of HFCs [6]. Additionally, the signing of the Paris Agreement (2016), which is committed to maintaining the global temperature rise within 2 °C, will further accelerate the phasing out process of HFCs.
In order to cope with the problem that no gas fire suppressant can be used in the future, perfluoro-2-methyl-3-pentanone (C6F12O), also known as FK-5112, Novec 1230 or Novec 649, with its environmentally friendly performance, zero ODP, GWP of approximately one and atmospheric lifetime of up to two weeks, has been considered as the next generation of halon alternatives. C6F12O belongs to fluorinated ketones which is different to HFCs. Its nontoxicity, noncombustibility, excellent insulation properties and fire suppression efficiency have attracted worldwide attention. It has been listed as an available substitute in the significant new alternatives policy (SNAP) program of the United States Environment Protection Agency (EPA) in 2003 and also listed in fire extinguishing system design standards including ISO 14520 and NFPA 2001. For now, C6F12O has been applied in many places such as electrical and electronic equipment, aircrafts, ships and libraries [7], and has shown great potential as a next generation halon substitute.
2. Synthesis Route
C6F12O is in great demand on the market with its excellent environmental performance and broad application prospects. It is of great significance to the development of a synthetic route for C6F12O, suitable for industrial production. There have been many reports on the synthesis of C6F12O, mainly in the following categories as shown in Figure 1.
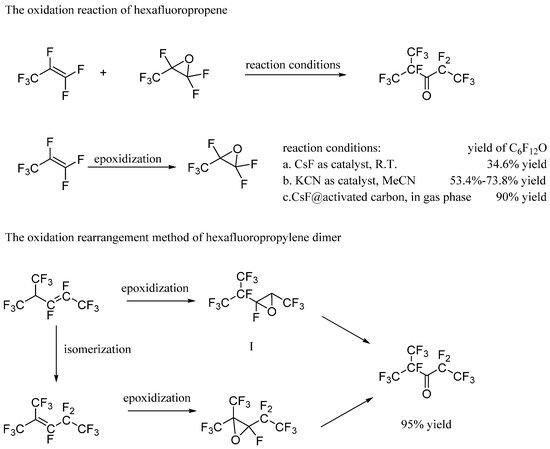
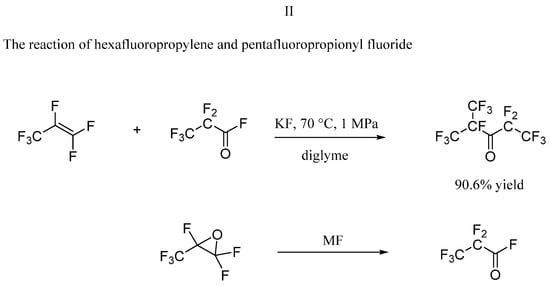
Figure 1. Three main synthesis routes for C6F12O.
2.1. The Oxidation Reaction of Hexafluoropropene
Vilenchik et al. [8] proposed that hexafluoropropene is oxidized to prepare hexafluoropropylene epoxide. Hexafluoropropylene epoxide could react with hexafluoropropene under room temperature catalyzed by cesium fluoride. This method with high molecular utilization but low reactive selectivity could give C6F12O, accounting for approximately 35%. Furthermore, the yield could be enhanced to approximately 50–70% using halogen-like potassium salts (i.e., KOCN, KSCN, KCN) as a catalyst and the acetonitrile as a solvent (SU698289). Hexafluoropropylene epoxide and hexafluoropropene could react through a gas phase reaction under a catalysis of cesium fluoride with activated carbon as the carrier, and the yield of C6F12O could be improved to more than 90% (RU2010150091A). However, the raw materials of this method are uncommon and the reaction yield in actual production is less than the theory. The by-product, hexafluoropropylene dimer, is difficult to separate and the reaction selectivity needs to be enhanced.
2.2. The Oxidation Rearrangement Method of Hexafluoropropylene Dimer
Zapevalov et al. [9] reported a method to prepare C6F12O through the oxidation rearrangement reaction for hexafluoropropylene dimer. Hexafluoropropylene dimer has two isomers including perfluoro-4-methyl-2-pentene and perfluoro-2-methyl-2-pentene. Additionally, two kinds of corresponding epoxides could be obtained from hexafluoropropylene dimer under the oxidation of sodium hypochlorite. The epoxides could be converted into C6F12O using cesium fluoride or trimethylamine, which is mild and could give 93% yield. This process includes multiple reactions, the yields of which need to be improved so that it could be practically used in the industry.
Compared with perfluoro-2,3-epoxy-4-methyl pentane, the epoxidation of perfluoro-2,3-epoxy-2-methyl pentane gives only one product, which is more suitable for the preparation of C6F12O. However, oligomerization of hexafluoropropylene mainly gives perfluoro-4-methyl-2-pentene. Additionally, it is now very significant in the industry to produce perfluoro-2-methyl-2-pentene through isomerization of perfluoro-4-methyl-2-pentene. The patent of 3M company (WO1995025082A1) disclosed a method for preparing C6F12O using perfluoro-2-methyl-2-pentene as a raw material through multi-step reactions, such as epoxidation by sodium hypochlorite and catalysis by cesium fluoride. Based on this, researchers (CN103508868B) have designed and optimized the reaction route: (1) isomerization from perfluoro-4-methyl-2-pentene to obtain perfluoro-2-methyl-2-pentene, (2) epoxidation to obtain perfluoro-2,3-epoxy-2-methyl pentane, and (3) catalysis to obtain C6F12O. In the optimization study of this method [10], the yield of C6F12O could reach more than 95%, which shows the prospective of the method. The patent (CN105198719B) disclosed a one-step preparation of C6F12O through the gas-phase reaction under the conditions of oxidizing gas and catalyst. This process requires only one step reaction with no solvent, reducing the pollution and cost.
2.3. The Reaction of Hexafluoropropylene and Perfluoropropionyl Fluoride
The reaction of hexafluoropropylene and perfluoropropionyl fluoride as raw materials under the conditions of alkali metal fluoride catalyst in aprotic polar solvent, diglyme or acetonitrile is also the main method for C6F12O synthesis [11]. 3M company (WO2001005468A2) disclosed the reaction conditions: diglyme as the solvent and active potassium fluoride as the catalyst, 70 °C, 1 MPa pressure in their patent. This reaction with high selectivity gives a C6F12O 90.6% yield. However, the raw material, perfluoropropionyl fluoride is highly corrosive, which is difficult to prepare and store. In addition, the reaction needs to be carried out under a higher pressure, and the entire process has high requirements on the material of the reactor. Fenichev et al. [12] proposed that perfluoropropionyl fluoride can be easily obtained by isomerization of hexafluoropropene oxide using alkali metal fluorides, KF and CsF, with the same conditions of C6F12O synthesis. They found that the water content in acetonitrile is the main factor resulting in the deactivation of the catalyst and the methods of isolating C6F12O from the reaction mixture by hydrolysis, and subsequent hydrogenation on the palladium catalyst supported on the activated carbon could lead the target product with a purity of 99.95 wt%. They also designed and created the pilot setup for production. Sha et al. [13] discussed the synthesis process of perfluoropropionyl fluoride. They produced hexafluoropropylene oxide through liquid phase oxidation of hexafluoropropylene as raw material using molecular oxygen, and then obtained the final product after isomerization using a bubbling reactor, which seems to be an ideal reaction route for industrial production.
All these three synthesis routes for C6F12O use hexafluoropropylene as the raw material. Some problems exist in these routes in that they all have certain side reactions, and the by-products are difficult to separate from major products. Moreover, the purity of the product should be paid more attention. There are various kinds of fluoride involved in the synthesis of C6F12O, and the toxicity of different fluorides varies greatly. If these substances are not effectively separated, even very small amounts of toxic impurities exist in the final product, the toxicity for the product of C6F12O will be totally different. It is urgent to optimize the synthesis routes and look for a safe, green, high yield, low in cost, less impurity and continuous synthesis routes for industrial production.
This entry is adapted from the peer-reviewed paper 10.3390/fire5020050
This entry is offline, you can click here to edit this entry!
