Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Antimicrobial multidrug-resistance (MDR) is an indirect consequence of large-scale and non-professional applications of previously powerful antibiotics, leading to the situation in which the lifesaving role of antibiotics has gradually become diminished. MDR has become not only a global public health concern, but also a challenge for sustainable agriculture and plant health management problems.
- antimicrobial multidrug resistance
1. Antimicrobial Peptides as Tools to Beat MDR Pathogens
The usage of the new arsenal of peptide antibiotics in the battle against multidrug-resistance (MDR) pathogens is of emerging therapeutic potential [1], since many newly appearing MDR organisms seem to show collateral sensitivity [2][3]. Furthermore, applying antimicrobial peptide (AMP) resistance and antibiotic resistance genes differ in their mobilization patterns and functional compatibilities with new bacterial hosts [4]. The various AMP molecules differ considerably concerning their physicochemical properties and cellular targets, as well as their resistance determinants [5]. Cross-resistance between AMPs appears to be rather rare [6]. Furthermore, the co-evolutionary trends of resistance against antimicrobial peptides [7], and those against conventional antibiotics, must also be different [6].
2. Changes in the Scope of the EPN/EPB Research Due to the Perspectives of EPB-Produced AMPs in Combatting MDR Pathogens
Similar to the research trends on entomopathogenic fungi [8][9], those related to entomopathogenic-nematode/bacterium (EPN/EPB) symbiotic associations have been restricted to biological insect pest control tools for sustainable agriculture [10][11][12][13][14][15][16][17][18][19][20][21][22].
The antibiotic-related perspectives were recognized [23] only when the global threat of MDR became obvious [24], although the antibiotic-productive capabilities of the obligate Gram-negative bacterial symbionts belonging to the Xenorhabdus and Photorhabdus genera of EPN strains, or belonging to species of the Steinernema and Heterorhabditis genera, had been known since 1972 [25][26][27][28][29].
Indispensable subchapters of the history of EPN/EPB research are those which revealed the detailed mechanisms and coevolutionary aspects of the symbioses [30][31], the unique unprecedented epigenetic mechanism called the primary/secondary (mostly) irreversible phenotypic phase shift both in Xenorhabdus and Photorhabdus [29][30][31][32][33], and the coevolutionary aspects [34][35][36][37][38][39][40][41][42][43][44].
3. How Do Antibiotic-Producing EPN/EPB Symbioses Work?
Insects, EPN, and EPB are capable of forming a tripartite relationship called mutualism [45]. This includes a host/parasite relationship between the EPN and the infested insect prey; a host/pathogen relationship between the colonized insect prey and the EPB pathogen; and finally a symbiotic relationship between the respective EPN and EPB [46], as demonstrated in Figure 1.
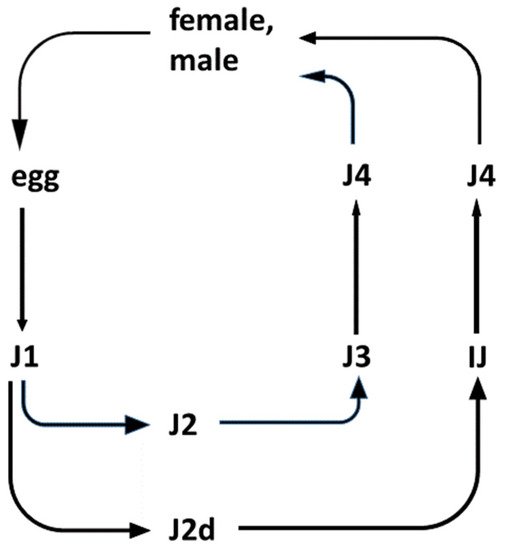
Figure 1. Life cycles of entomopathogenic Steinernema nematode species. (The life-cycle of Heterorhabditis is not shown). Legend: The outlines of the life-cycle of EPN species belonging to the Steinernema genus. There are six postembryonic developmental (juvenile, larval) stages (J1, J2, J2d, J3, J4, and IJ: J = juvenile; I = infective; and IJ = infective dauer, or the enduring, non-aging, non-feeding, non-growing, semi-anabiotic larva [47]. In nature, only the IJ can be found in the soil outside the insect cadaver. They are capable of entering the insects through their natural openings, and infecting them. Immediately after entry into the insect, the pharynx of the IJ starts to pump, releasing their symbiotic bacteria through their mouths into the hemocoel of the insect. The bacteria propagate rapidly and release toxins, killing the insect host. Meanwhile, the IJ molts, and develops into a J4. Adults develop from the J4 in the insect cadaver. In the case of Steinernema, the adults are 50/50 female/male. Males then fertilize the females. In the case of Heterorhabditis, self-fertilizing adult hermaphrodites develop from the infecting IJ. Most of their eggs develop inside the hermaphrodite (called “endotoxin matricida”), and the majority develop into females and males. Only a small fraction grows to additional self-fertilizing hermaphrodites. After 2–3 cycles, the concentration of a secondary metabolite of a lipid nature, which serves as a genus-specific chemical developmental signal, reaches a level that induces an altered developmental pathway for the J1 larvae to develop to an IJ through a special second (J2d) developmental stage. IJs leave the cadaver and search for new insect hosts, aided by chemo-attraction.
The EPN/EPB symbiosis is taxon-specific. Whereas the Steinernema EPNs can only establish symbiosis with bacteria belonging to the genus Xenorhabdus, EPNs in genus Heterorhabditis can only establish symbiosis with Photorhabdus bacteria. The dauer juvenile (IJ) nematodes store, with few known exceptions, the respective symbiont monoxenically in their guts. The feeding forms (J1, J2, J2d, J3, J4, and adults) consume the bacteria together with the bacterium-digested insect tissues.
4. The Natural Role of the EPB
As mentioned in the introduction, the biological role of antimicrobial, mainly peptide, products of the EPB is to provide, establish and sustain monoxenic or balanced pathobiome conditions for the respective nematode/bacterium symbiotic complex in a polyxenic environment, such as the cadaver of the vanquished insect in the soil, in the niche where they live [31][32][48]. More accurately, during the long-term observations, whenever the EPB was isolated in sterile conditions from the gut of surface sterilized IJs [49], it found only one single bacterium species, and this was the respective EPB symbiont in the primary (phase I, 1) form, in agreement with [31][32], see Figure 2.
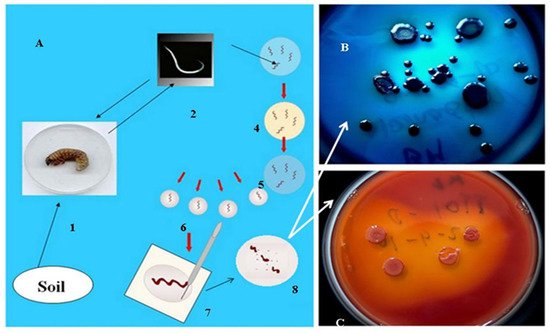
Figure 2. Isolation of EPB cells from IJs (A) by Lucskai’s bleach method. Infective dauer juveniles, either from the soil, or insect cadaver, are surface sterilized in HOCL, put in a sterile physiological salt solution in a sterile Petri dish, cut into pieces, and μL volumes from the saline solution are dropped in agar (LBA) media, (C) seeded, and incubated at 25 °C for one day. Fresh colonies are picked and transferred to an indicator (LBTA) plate (B) where the Phase I DPN cells can be unambiguously recognized and cloned.
However, whenever EPB were attempted to be isolated from an infected insect cadaver, they were never found them monoxenically, in agreement with [48].
The triple role of the EPB symbiont is as follows: (1) producing insect-killing toxins [50][51][52], (“serving like a soldier”); (2) digesting the insect tissues making them consumable for the EPN, (“acting like a cook”); and (3) as a producer of antimicrobial peptides to protect the EPN/EPB symbiotic complex from competitors existing in the polyxenic soil, (“serving like a bodyguard”, [25][26][27][28]). This is schematically summarized in Figure 3.
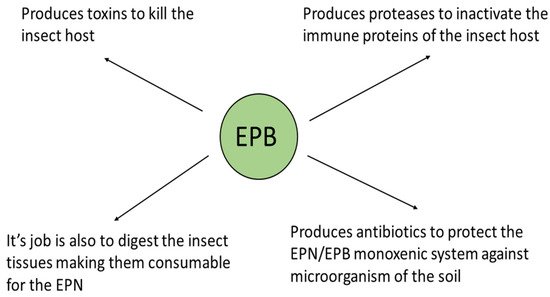
Figure 3. The biological role of the EPB symbiont in nature. (Presented by Fodor et al. BABE-2015 6th World Congress on Bioavailability & Bioequivalence: BA/BE Studies Summit 17–19 August 2015).
Immediately after entering the insect body cavity through the natural openings of the insect, the IJ releases the EPB into the hemocoel, where it starts to propagate and produce toxins of a protein nature, causing lethal septicemia in the insect prey, and decomposing the insect tissue. This tissue becomes consumable for the propagating EPN population, and releases antimicrobials to provide balanced, probiotic, conditions for the symbiotic complex, the polyxenic cadaver, and the soil [48].
This entry is adapted from the peer-reviewed paper 10.3390/pathogens11030342
References
- Upert, G.; Luther, A.; Obrecht, D.; Ermert, P. Emerging peptide antibiotics with therapeutic potential. Med. Drug Discov. 2021, 9, 100078.
- Pál, C.; Papp, B.; Lázár, V. Collateral sensitivity of antibiotic-resistant microbes. Trends Microbiol. 2015, 23, 401–407.
- Lázár, V.; Martins, A.; Spohn, R.; Daruka, L.; Grézal, G.; Fekete, G.; Számel, M.; Jangir, P.K.; Kintses, B.; Csörgő, B.; et al. Antibiotic-resistant bacteria show widespread collateral sensitivity to antimicrobial peptides. Nat. Microbiol. 2018, 3, 718–731.
- Kintses, B.; Méhi, O.; Ari, E.; Számel, M.; Györkei, Á.; Jangir, P.K.; Nagy, I.; Pál, F.; Fekete, G.; Tengölics, R.; et al. Phylogenetic barriers to horizontal transfer of antimicrobial peptide resistance genes in the human gut microbiota. Nat. Microbiol. 2019, 4, 447–458.
- Kintses, B.; Jangir, P.K.; Fekete, G.; Számel, M.; Méhi, O.; Spohn, R.; Daruka, L.; Martins, A.; Hosseinnia, A.; Gagarinova, A.; et al. Chemical-genetic profiling reveals limited cross-resistance between antimicrobial peptides with different modes of action. Nat. Commun. 2019, 10, 5731.
- Fodor, A.; Abate, B.A.; Deák, P.; Fodor, L.; Gyenge, E.; Klein, M.G.; Koncz, Z.; Muvevi, J.; Ötvös, L.; Székely, G.; et al. Multidrug resistance (MDR) and collateral sensitivity in bacteria, with special attention to genetic and evolutionary aspects and to the perspectives of antimicrobial peptides-A Review. Pathogens 2020, 9, 522.
- Baindara, P.; Ghosh, A.K.; Mandal, S.M. Coevolution of resistance against antimicrobial peptides. Microb. Drug Resist. 2020, 26, 880–899.
- Khun, K.K.; Wilson, B.A.L.; Stevens, M.M.; Huwer, R.K.; Ash, G.J. Integration of entomopathogenic fungi into IPM programs: Studies involving weevils (Coleoptera: Curculionoidea) affecting horticultural crops. Insects 2020, 1, 659.
- Mann, A.J.; Davis, T.S. Entomopathogenic fungi to control bark beetles: A review of ecological recommendations. Pest Manag. Sci. 2021, 77, 3841–3846.
- Thomas, G.M.; Poinar, G.O., Jr. A new bacterium, Achromobacter nematophilus sp. nov. (Achromobacteriaceae: Eubacteriales) associated with a nematode. Int. J. Syst. Evol. Microbiol. 1965, 15, 249–254.
- Poinar, G.O., Jr.; Thomas, G.M. (Achromobacteraceae: Eubacteriales) in the development of the nematode, DD-136 (Neoaplectana sp. Steinernematidae). Parasitology 1966, 56, 385–390.
- Kaya, H.K. Development of the DD-136 strain of Neoaplectana carpocapsae at constant temperatures. J. Nematol. 1977, 9, 346–349.
- Poinar, G.O., Jr.; Thomas, G.M.; Hess, R. Characteristics of the specific bacterium associated with Heterorhabditis bacteriophora (Heterorhabditidae: Rhabditida). Nematologica 1977, 23, 97–102.
- Thomas, G.M.; Poinar, G.O., Jr. Xenorhabdus gen. nov., a genus of entomopathogenic nematophilic bacteria of the family Enterobacteriaceae. Int. J. Syst. Bacteriol. 1979, 29, 352–360.
- Bedding, R.A.; Molyneux, A.S.; Akhurst, R.J. Heterorhabditis spp., Neoaplectana spp., and Steinernema kraussei: Interspecific and intraspecific differences in infectivity for insects. Exp. Parasitol. 1983, 55, 249–257.
- Ehlers, R.U. Mass production of entomopathogenic nematodes for plant protection. Appl. Microbiol. Biotechnol. 2001, 56, 623–633.
- Samish, M.; Glazer, I. Entomopathogenic nematodes for the biocontrol of ticks. Trends Parasitol. 2001, 17, 368–371.
- Shapiro-Ilan, D.I.; Gaugler, R. Production technology for entomopathogenic nematodes and their bacterial symbionts. J. Ind. Microbiol. Biotechnol. 2002, 28, 137–146.
- Piñero, J.C.; Shapiro-Ilan, D.; Cooley, D.R.; Tuttle, A.F.; Eaton, A.; Drohan, P.; Leahy, K.; Zhang, A.; Hancock, T.; Wallingford, A.K.; et al. Toward the integration of an Attract-and-Kill approach with entomopathogenic nematodes to control multiple life stages of plum curculio (Coleoptera: Curculionidae). Insects 2020, 11, 375.
- Torrini, G.; Paoli, F.; Mazza, G.; Simoncini, S.; Benvenuti, C.; Strangi, A.; Tarasco, E.; Barzanti, G.P.; Bosio, G.; Cutino, I.; et al. Evaluation of indigenous entomopathogenic nematodes as potential biocontrol agents against Popillia japonica (Coleoptera: Scarabaeidae) in Northern Italy. Insects 2020, 11, 804.
- Fanelli, E.; Troccoli, A.; Tarasco, E.; De Luca, F. Molecular characterization and functional analysis of the Hb-hsp90-1 gene in relation to temperature changes in Heterorhabditis bacteriophora. Front. Physiol. 2021, 12, 615653.
- Tarasco, E.; De Luca, F. Biological control and insect pathology. Insects 2021, 12, 291.
- Bode, H.B. Entomopathogenic bacteria as a source of secondary metabolites. Curr. Opin. Chem. Biol. 2009, 13, 224–230.
- Boucher, H.W.; Talbot, G.H.; Bradley, J.S.; Edwards, J.E.; Gilbert, D.; Rice, L.B.; Scheld, M.; Spellberg, B.; Bartlett, J. Bad bugs, no drugs: No ESKAPE! An update from the Infectious Diseases Society of America. Clin. Infect. Dis. 2009, 48, 1–12.
- Nealson, K.H.; Eberhard, A.; Hastings, J.W. Catabolite repression of bacterial bioluminescence: Functional implications. Proc. Natl. Acad. Sci. USA. 1972, 69, 1073–1076.
- Paul, W.J.; Frautschv, S.; Fenical, W.; Nealson, K.H. Antibiotics in microbial ecology: Isolation and structure assignment of several new antibacterial compounds for the insect symbiotic bacteria, Xenorhabdus spp. J. Chem. Ecol. 1981, 7, 589–597.
- Akhurst, R.J. Antibiotic activity of Xenorhabdus spp., bacteria symbiotically associated with insect pathogenic nematodes of the families Heterorhabditidae and Steinernematidae. J. Gen. Microbiol. 1982, 128, 3061.
- Akhurst, R.J. Neoaplectana species: Specificity of association with bacteria of the genus Xenorhabdus. Exp. Parasitol. 1983, 55, 258–263.
- Akhurst, R.J.; Boemare, N.E. A numerical taxonomic study of the genus Xenorhabdus (Enterobacteriaceae) and proposed elevation of the subspecies of X. nematophilus to species. J. Gen. Microbiol. 1988, 134, 1835–1845.
- Smigielski, A.J.; Akhurst, R.J.; Boemare, N.E. Phase variation in Xenorhabdus nematophilus and Photorhabdus luminescens: Differences in respiratory activity and membrane energization. Appl. Environ. Microbiol. 1994, 60, 120–125.
- Forst, S.; Nealson, K. Molecular biology of the symbiotic-pathogenic bacteria Xenorhabdus spp. and Photorhabdus spp. Microbiol. Rev. 1996, 60, 21–43.
- Forst, S.; Dowds, B.; Boemare, N.; Stackebrandt, E. Xenorhabdus and Photorhabdus spp.: Bugs that kill bugs. Annu. Rev. Microbiol. 1997, 51, 47–72.
- Völgyi, A.; Fodor, A.; Szentirmai, A.; Forst, S. Phase variation in Xenorhabdus nematophilus. Appl. Environ. Microbiol. 1998, 64, 1188–1193.
- Szállás, E.; Koch, C.; Fodor, A.; Burghart, J.; Buss, O.; Szentirmai, A.; Nealson, K.H.; Stackebrandt, E. Phylogenetic evidence for the taxonomic heterogeneity of Photorhabdus luminescens. Int. J. Syst. Bacteriol. 1997, 47, 402–407.
- Szállás, E.; Pukall, R.; Pamjav, H.; Kovács, G.; Buzás, Z.; Fodor, A.; Stackebrandt, E. Passengers who missed the train: Comparative sequence analysis, PhastSystem PAGE-PCR-RFLP and automated RiboPrint Phenotypes of Photorhabdus strains In Development in Entomopathogenic Nematode/Bacterial Research; Griffin, C.T., Burnell, A.M., Downes, M.J., Mulder, R., Eds.; European Commission Publications: Luxemburg, 2001; pp. 36–53.
- de Soete, G. A least square algorithm for fitting additive trees to proximity data. Psychometrika 1983, 48, 621–626.
- Munro, H.N. (Ed.) Jukes and Cantor, Evolution of protein molecules. In Mammalian Protein Metabolism; Academic Press: New York, NY, USA, 1969; pp. 21–132.
- Felsenstein, J. PHYLIP Phylogeny Inference Package, Version 3.5.1; Department of Genetics, University of Washington: Seattle, WA, USA, 1993.
- Triga, D.; Pamjav, H.; Vellai, T.; Fodor, A.; Buzás, Z. Gel electrophoretic restriction fragment length polymorphism analysis of DNA derived from individual nematodes, using the PhastSystem. Electrophoresis 1999, 20, 1274–1279.
- Pamjav, H.; Triga, D.; Buzás, Z.; Vellai, T.; Lucskai, A.; Adams, B.; Reid, A.P.; Burnell, A.; Griffin, C.; Glazer, I.; et al. Novel application of PhastSystem polyacrylamide gel electrophoresis using restriction fragment length polymorphism--internal transcribed spacer patterns of individuals for molecular identification of entomopathogenic nematodes. Electrophoresis 1999, 20, 1266–1273.
- Tailliez, P.; Pagès, S.; Ginibre, N.; Boemare, N. New insight into diversity in the genus Xenorhabdus, including the description of ten novel species. Int. J. Syst. Evol. Microbiol. 2006, 56, 2805–2818.
- Böszörményi, E. Entomopathogen Bacterium Antibiotic Activity and Symbiotic Capacity of Gnotobiological Analyses. Ph.D Thesis, Eötvös University, Budapest, Hungary, 2010. Available online: http://teo.elte.hu/minosites/tezis2010/burgettine_boszormenyi_e.pdf (accessed on 1 August 2019).
- Tailliez, P.; Laroui, C.; Ginibre, N.; Paule, A.; Pagès, S.; Boemare, N. Phylogeny of Photorhabdus and Xenorhabdus based on universally conserved protein-coding sequences and implications for the taxonomy of these two genera. Proposal of new taxa: X. vietnamensis sp. nov., P. luminescens subsp. caribbeanensis subsp. nov., P. luminescens subsp. hainanensis subsp. nov., P. temperata subsp. khanii subsp. nov., P. temperata subsp. tasmaniensis subsp. nov., and the reclassification of P. luminescens subsp. thracensis as P. temperata subsp. thracensis comb. nov. Int. J. Syst. Evol. Microbiol. 2010, 60, 1921–1937.
- Machado, R.A.R.; Wüthrich, D.; Kuhnert, P.; Arce, C.C.M.; Thönen, L.; Ruiz, C.; Zhang, X.; Robert, C.A.M.; Karimi, J.; Kamali, S.; et al. Whole-genome-based revisit of Photorhabdus phylogeny: Proposal for the elevation of most Photorhabdus subspecies to the species level and description of one novel species Photorhabdus bodei sp. nov., and one novel subspecies Photorhabdus laumondii subsp. clarkei subsp. nov. Int. J. Syst. Evol. Microbiol. 2018, 68, 2664–2681.
- Goodrich-Blair, H.; Clarke, D.J. Mutualism and pathogenesis in Xenorhabdus and Photorhabdus: Two roads to the same destination. Mol. Microbiol. 2007, 64, 260–268.
- Morran, L.T.; Penley, M.J.; Byrd, V.S.; Meyer, A.J.; O’Sullivan, T.S.; Bashey, F.; Goodrich-Blair, H.; Lively, C.M. Nematode-bacteria mutualism: Selection within the mutualism supersedes selection outside of the mutualism. Evolution 2016, 70, 687–695.
- Cassada, R.C.; Russell, R.L. The dauerlarva, a post-embryonic developmental variant of the nematode Caenorhabditis elegans. Dev. Biol. 1975, 46, 326–342.
- Ogier, J.C.; Pagès, S.; Frayssinet, M.; Gaudriault, S. Entomopathogenic nematode-associated microbiota: From monoxenic paradigm to pathobiome. Microbiome 2020, 8, 25.
- Lengyel, K.; Lang, E.; Fodor, A.; Szállás, E.; Schumann, P.; Stackebrandt, E. Description of four novel species of Xenorhabdus, family Enterobacteriaceae: Xenorhabdus budapestensis sp. nov., Xenorhabdus ehlersii sp. nov., Xenorhabdus innexi sp. nov., and Xenorhabdus szentirmaii sp. nov. Syst. Appl. Microbiol. 2005, 28, 115–122, Erratum in: Syst. Appl. Microbiol. 2007, 30, 83.
- ffrench-Constant, R.; Bowen, D. Photorhabdus toxins: Novel biological insecticides. Curr. Opin. Microbiol. 1999, 2, 284–288.
- ffrench-Constant, R.; Dowling, A.; Waterfield, N.R. Insecticidal toxins from Photorhabdus bacteria and their potential use in agriculture. Toxicon 2007, 49, 436–451.
- Kajla, M.K. Symbiotic bacteria as potential agents for mosquito control. Trends Parasitol. 2020, 36, 4–7.
This entry is offline, you can click here to edit this entry!
