Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Antimicrobial multidrug-resistance (MDR) is an indirect consequence of large-scale and non-professional applications of previously powerful antibiotics, leading to the situation in which the lifesaving role of antibiotics has gradually become diminished. MDR has become not only a global public health concern, but also a challenge for sustainable agriculture and plant health management problems.
- antimicrobial multidrug resistance
1. Antimicrobial Peptides as Tools to Beat MDR Pathogens
The usage of the new arsenal of peptide antibiotics in the battle against multidrug-resistance (MDR) pathogens is of emerging therapeutic potential [1], since many newly appearing MDR organisms seem to show collateral sensitivity [2][3]. Furthermore, applying antimicrobial peptide (AMP) resistance and antibiotic resistance genes differ in their mobilization patterns and functional compatibilities with new bacterial hosts [4]. The various AMP molecules differ considerably concerning their physicochemical properties and cellular targets, as well as their resistance determinants [5]. Cross-resistance between AMPs appears to be rather rare [6]. Furthermore, the co-evolutionary trends of resistance against antimicrobial peptides [7], and those against conventional antibiotics, must also be different [6].
2. Changes in the Scope of the EPN/EPB Research Due to the Perspectives of EPB-Produced AMPs in Combatting MDR Pathogens
Similar to the research trends on entomopathogenic fungi [8][9], those related to entomopathogenic-nematode/bacterium (EPN/EPB) symbiotic associations have been restricted to biological insect pest control tools for sustainable agriculture [10][11][12][13][14][15][16][17][18][19][20][21][22].
The antibiotic-related perspectives were recognized [23] only when the global threat of MDR became obvious [24], although the antibiotic-productive capabilities of the obligate Gram-negative bacterial symbionts belonging to the Xenorhabdus and Photorhabdus genera of EPN strains, or belonging to species of the Steinernema and Heterorhabditis genera, had been known since 1972 [25][26][27][28][29].
Indispensable subchapters of the history of EPN/EPB research are those which revealed the detailed mechanisms and coevolutionary aspects of the symbioses [30][31], the unique unprecedented epigenetic mechanism called the primary/secondary (mostly) irreversible phenotypic phase shift both in Xenorhabdus and Photorhabdus [29][30][31][32][33], and the coevolutionary aspects [34][35][36][37][38][39][40][41][42][43][44].
3. How Do Antibiotic-Producing EPN/EPB Symbioses Work?
Insects, EPN, and EPB are capable of forming a tripartite relationship called mutualism [45]. This includes a host/parasite relationship between the EPN and the infested insect prey; a host/pathogen relationship between the colonized insect prey and the EPB pathogen; and finally a symbiotic relationship between the respective EPN and EPB [46], as demonstrated in Figure 1.
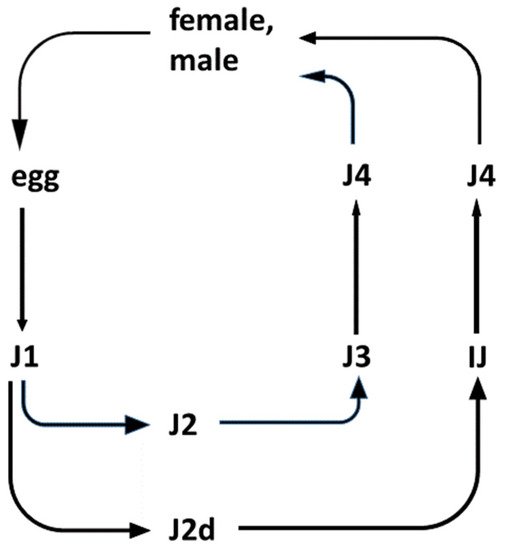
Figure 1. Life cycles of entomopathogenic Steinernema nematode species. (The life-cycle of Heterorhabditis is not shown). Legend: The outlines of the life-cycle of EPN species belonging to the Steinernema genus. There are six postembryonic developmental (juvenile, larval) stages (J1, J2, J2d, J3, J4, and IJ: J = juvenile; I = infective; and IJ = infective dauer, or the enduring, non-aging, non-feeding, non-growing, semi-anabiotic larva [47]. In nature, only the IJ can be found in the soil outside the insect cadaver. They are capable of entering the insects through their natural openings, and infecting them. Immediately after entry into the insect, the pharynx of the IJ starts to pump, releasing their symbiotic bacteria through their mouths into the hemocoel of the insect. The bacteria propagate rapidly and release toxins, killing the insect host. Meanwhile, the IJ molts, and develops into a J4. Adults develop from the J4 in the insect cadaver. In the case of Steinernema, the adults are 50/50 female/male. Males then fertilize the females. In the case of Heterorhabditis, self-fertilizing adult hermaphrodites develop from the infecting IJ. Most of their eggs develop inside the hermaphrodite (called “endotoxin matricida”), and the majority develop into females and males. Only a small fraction grows to additional self-fertilizing hermaphrodites. After 2–3 cycles, the concentration of a secondary metabolite of a lipid nature, which serves as a genus-specific chemical developmental signal, reaches a level that induces an altered developmental pathway for the J1 larvae to develop to an IJ through a special second (J2d) developmental stage. IJs leave the cadaver and search for new insect hosts, aided by chemo-attraction.
The EPN/EPB symbiosis is taxon-specific. Whereas the Steinernema EPNs can only establish symbiosis with bacteria belonging to the genus Xenorhabdus, EPNs in genus Heterorhabditis can only establish symbiosis with Photorhabdus bacteria. The dauer juvenile (IJ) nematodes store, with few known exceptions, the respective symbiont monoxenically in their guts. The feeding forms (J1, J2, J2d, J3, J4, and adults) consume the bacteria together with the bacterium-digested insect tissues.
4. The Natural Role of the EPB
As mentioned in the introduction, the biological role of antimicrobial, mainly peptide, products of the EPB is to provide, establish and sustain monoxenic or balanced pathobiome conditions for the respective nematode/bacterium symbiotic complex in a polyxenic environment, such as the cadaver of the vanquished insect in the soil, in the niche where they live [31][32][48]. More accurately, during the long-term observations, whenever the EPB was isolated in sterile conditions from the gut of surface sterilized IJs [49], it found only one single bacterium species, and this was the respective EPB symbiont in the primary (phase I, 1) form, in agreement with [31][32], see Figure 2.
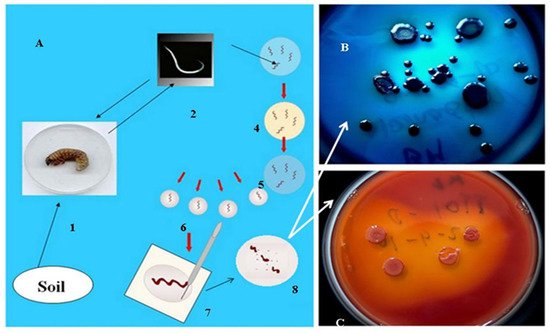
Figure 2. Isolation of EPB cells from IJs (A) by Lucskai’s bleach method. Infective dauer juveniles, either from the soil, or insect cadaver, are surface sterilized in HOCL, put in a sterile physiological salt solution in a sterile Petri dish, cut into pieces, and μL volumes from the saline solution are dropped in agar (LBA) media, (C) seeded, and incubated at 25 °C for one day. Fresh colonies are picked and transferred to an indicator (LBTA) plate (B) where the Phase I DPN cells can be unambiguously recognized and cloned. (For details, see Appendix A).
However, whenever EPB were attempted to be isolated from an infected insect cadaver, they were never found them monoxenically, in agreement with [48].
The triple role of the EPB symbiont is as follows: (1) producing insect-killing toxins [50][51][52], (“serving like a soldier”); (2) digesting the insect tissues making them consumable for the EPN, (“acting like a cook”); and (3) as a producer of antimicrobial peptides to protect the EPN/EPB symbiotic complex from competitors existing in the polyxenic soil, (“serving like a bodyguard”, [25][26][27][28]). This is schematically summarized in Figure 3.
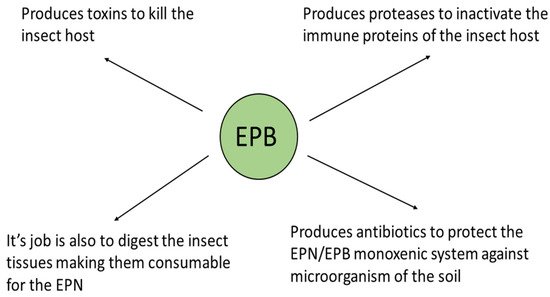
Figure 3. The biological role of the EPB symbiont in nature. (Presented by Fodor et al. BABE-2015 6th World Congress on Bioavailability & Bioequivalence: BA/BE Studies Summit 17–19 August 2015).
Immediately after entering the insect body cavity through the natural openings of the insect, the IJ releases the EPB into the hemocoel, where it starts to propagate and produce toxins of a protein nature, causing lethal septicemia in the insect prey, and decomposing the insect tissue. This tissue becomes consumable for the propagating EPN population, and releases antimicrobials to provide balanced, probiotic, conditions for the symbiotic complex, the polyxenic cadaver, and the soil [48].
This entry is adapted from the peer-reviewed paper 10.3390/pathogens11030342
This entry is offline, you can click here to edit this entry!
