Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Health Care Sciences & Services
In practical terms, lung ultrasound (LUS) can be considered as an equally accurate alternative for CT in many situations where CT is not easily accessible or when molecular tests are not available. The use of lung ultrasound (LUS) as a triage tool has been proposed since the beginning of the COVID-19 pandemicand subsequent studies have confirmed its role. The high sensitivity of ultrasound for the superficial lesions of the lung from the interstitial stages represents its great value.
- COVID-19
- clinical review
- lung ultrasound imaging
1. Clinical Interpretation of SIS
SIS is a non-specific echographic pattern. As it appears in various pathologies of the lung, its interpretation must include further information relating to its appearance and clinical context. Similarly, ultrasound COVID-19 findings, being also based on the presence of SIS, are not specific in themselves. To increase the specificity of ultrasound, when approaching SIS, it is important to focus on four steps in every situation:
-
Characteristics of the pleural line;
-
Characteristics of the artifacts;
-
Extension and distribution of SIS;
-
Relationships with clinical data and integrated multi-district sonography.
1.1. Characteristics of the Pleural Line
Once an US pulse reaches the visceral pleural surface it is near-totally reflected by the non-diseased lung because the size of the intra and interalveolar septa are relatively thin (with respect to the wave length of the carrier frequency). In these cases, the US pulse meets a sort of air wall, it is near-totally reflected towards the probe and the outer lung surface is represented as a thick white line where the thickness of this line is related to the length of the US pulse. It is necessary, here, to highlight that the perceived thickness of the pleural line is, in theory, equal to the length of the US pulse. However, this is true only if the direction of the wave propagation is orthogonal to the pleural plane. When the insonation is not orthogonal, and, even more importantly, when the visceral pleura is not “healthy” (in the presence of slightly thickened interstitial spaces, for example), it can appear blurred and apparently thickened.
In cases where the pleura is not a good acoustic reflector, even in the absence of vertical artifacts, the signs to be taken into consideration are the blurred and thickened appearance of the pleural line and, as a consequence, the less evident replica and mirror effects of the parietal structures below it.
The above is in agreement with known clinical experience. In ACPE, the pleural line is regular, smooth, linear, and with normal sliding. Generally, in primitive pulmonary interstitial diseases, the pleura contributes to the generation of artifacts, and is stably irregular, cobbled or even finely interrupted, especially in the basal regions [26,27,28]. Typical signs of ARDS are spared areas, and a normal or poorly altered pleural line with normal sliding next to areas with irregular pleura, which shows reduced or absent movements [29,30,31]. The pleural line is slight and focally irregular in score 1 (see Section 4) COVID-19, and becomes progressively more irregular as the disease progresses.
1.2. Artifacts’ Characteristics
Different vertical artifacts are observed in acute cardiogenic pulmonary edema (ACPE), and acute respiratory distress syndrome (ARDS) or pulmonary fibrosis [3,29] .
In the case of ACPE (especially early ACPE), the lung architecture remains unchanged and shows only septal enlargement by transudate. The artifacts in early pulmonary edema are B-lines with their characteristic pleural point-like origin and brightness. In contrast, in pulmonary fibrosis, vertical artifacts are variable in morphology and distribution, and are often distinguishable by their low level of brightness and rapid attenuation.
In ARDS, the artefactual pattern is pneumogenic, inhomogeneous, and typically gravitational, with the most aerated lung in an elevated position and the denser or consolidated lung in the sloping position [32].
In COVID-19, a distribution of artifacts similar to that seen in early ARDS is present, but many single B-lines are brighter, and patchy columnar areas of white lung can be seen.
1.3. Extension and Distribution
SIS can be either focal, multifocal, or diffuse [27]. Mono- or oligofocal SIS is often seen around monolateral pulmonary consolidation, representing a denser but not consolidated tissue. This finding is suggestive of bacterial pneumonia.
When SIS is diffuse and bilateral, it is indicative of a diffuse pulmonary pathology. It can be either homogeneous or inhomogeneous. Homogeneous SIS can show a gravitational distribution, without spared areas. This could be indicative of cardiogenic pulmonary edema [12]. When SIS is bilateral and inhomogeneous, with spared areas, it could be indicative of non-cardiogenic pathology [22,26]. SIS in COVID-19 patients is typically pneumogenic and appears inhomogeneous and patchy, and is more prevalent in the basal portions of the lungs.
1.4. Relationships with Clinical Data and Integrated Multi-District Sonography
Given the non-specificity of vertical artifacts, it is only possible to suspect that one condition is more probable than another through an inferential abductive process of reasoning, which allows clinicians to make a more accurate diagnosis [1].
Knowledge of the clinical history and the preclinical probability of disease is useful and becomes crucial in the event of a COVID-19 epidemic. In the case of suspected cardiogenic SIS, echocardiography can add much information (cardiac signs of diastolic/systolic heart failure) [29]. Inferior caval vein dynamics give a rough preload estimate. A multi-district approach is useful when many diseases co-occur to establish a clinical picture or when cardiac or renal complications occur in COVID-19 patients.
In the case of diffuse pneumogenic SIS associated with fibrotic interstitial lung diseases (ILDs), a typical appearance and distribution of artifacts with a congruent clinical picture (non-epidemic, subacute, or chronic onset) can aid in the diagnosis. Creating an acoustic pulmonary map is useful for narrowing down the diagnostic options, especially in diffuse ILDs [31].
2. Clinical Basis of COVID-19 Lung Ultrasound Imaging
Coronavirus disease 2019 (COVID-19) appeared in Wuhan, China, in December 2019, and rapidly increased to a pandemic level around the world. Its etiological agent is a novel coronavirus named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [33]. In the previous two decades, coronaviruses have caused other epidemic diseases—SARS-CoV-1 and Middle East respiratory syndrome (MERS-CoV). In SARS-CoV-1 coronavirus pneumonia, peripheral lung involvement was common but unifocal involvement was more common than multifocal or bilateral involvement. On CT images, GGOs with consolidations were the main findings, and reticulation was noted after the second week [34]. On CT images, MERS-CoV pneumonia showed subpleural and basilar airspace lesions, with extensive subpleural GGO and consolidation. Studies concerning the use of US in SARS-CoV-1 and in MERS-CoV are lacking. However, on the basis of the CT findings, we can suppose that the ultrasound signs in these pathologies would have been similar to those in patients with COVID-19. COVID-19, SARS-CoV-1, and MERS-CoV cause lung damage and, in their final stages, also multiorgan failure [35].
In general, in COVID-19 the most serious initial symptoms are related to pneumonia, while the evolution towards respiratory failure is similar to ARDS [36]. In light of our current knowledge regarding COVID-19, this picture may be too simplistic.
Many viruses cause pneumonia. Histopathology of viral pneumonia varies, and is related to the pathogenesis of pulmonary infection. Consequently, a computed tomographic (CT) pattern of viral pneumonia reports, at best, the fine pathology at the lobular level. Generally, interstitial viral pneumonitis shows a thickened interstitium with lymphocytic infiltration, and viral particles can be seen in both the bronchial and alveolar epithelium. Hyperplasia and desquamation of the alveolar lining cells and hemorrhage are the result of the harmful action of some viruses [37]. Histopathological findings in cases of SARS-CoV-1 and influenza infection (H1N1, H5N1) are characterized by diffuse alveolar damage (DAD), hemorrhage, edema, and hyaline membrane [31].
The histopathological picture of initial COVID-19 involvement is characterized by patchy DAD, interstitial thickness, and pneumocyte hyperplasia. Late stages show alveolar congestion, desquamation, organizing pneumonia, and hemorrhage [38,39,40]. More recent papers pay attention to the thickening of alveolar capillaries surrounded by edema, intraluminal fibrin thrombi, and CD61+ megakaryocytes in association with platelets [41]. Despite a direct viral infection of the endothelial cells being reported, other mechanisms of vascular involvement have been proposed. Magro et al. [42] examined lung tissue from five COVID-19 patients with respiratory failure. Histopathological patterns were characterized by significant capillary fibrin deposition. Vascular deposits of terminal complement components (C5-b9, C4d, MASP2) were noted, suggesting a systemic activation of a lectin-based complement pathway. Therefore, a complement-mediated coagulative dysregulation is possible. The proportion of COVID-19 patients with abnormal initial radiographic findings is low (50% or less). In these patients, chest computed tomography (CT) has a high sensitivity (97%) but a lower specificity (56%) for lung involvement, showing subpleural patchy ground-glass opacities (GGO), reticular and crazy paving patterns, and finally consolidations. A study demonstrated that lung involvement gradually increased to consolidation up to two weeks after the onset of the disease (72%, half of these with subsegmental appearance). In general, consolidations are considered an indication of disease progression [43,44].
When interpreting the lung ultrasound findings related to COVID-19, both the structural variations of the superficial lung tissue and CT findings are important. The former defines the appearance of the ultrasound images while the latter their sonographic visibility (only superficial alterations are visible with LUS).
In CT, GGO are present in 100% of cases, appearing in peripheral locations in 89% of cases. While 93% of patients have multilobar and posterior lung involvement, 91% of patients have bilateral findings [43]. These characteristics allow us to indicate ultrasound as a diagnostic tool in COVID-19 lung involvement. In accordance with the physical basis of the formation of ultrasound images, reticular and ground-glass opacities appear as artifacts, while the consolidative ones are anatomical tissue images [13,45].
Ultrasound reproduces superficial consolidations in explorable thoracic regions with excellent accuracy, including the presence of air and fluid bronchograms. Consolidations, that are evident in CT, appear on ultrasound if they emerge from the pleura. CT superficial interstitial thickenings appear on ultrasound as small consolidations or as vertical artifacts with variable lengths and modulations in relation to their shape and size. Ground-glass CT typically appears as white lung [3,4,13].
3. SIS in COVID-19
SIS is an expected finding in COVID-19 because edema, DAD, lung hemorrhage, interstitial thickening, hyaline membranes, and non-consolidative infiltrative lung diseases (if abutting the pleura) generate artefactual signs in LUS. In physical terms, the common denominator of these conditions is an increase in the density of the involved lung areas compared to the healthy lung [15]. The topographical distribution of COVID-19 findings, as visible in CT, justifies their appearance and the typically bilateral, multilobar, and patchy pattern [1]. The most affected lung areas are the posteroinferior (93.8%) followed by the lateral (88.7%) [46].
Many studies have addressed lung ultrasound findings in COVID-19 patients. Most of these adapted past experiences in other fields (intensive-care medicine and ARDS-CoV-1) to COVID-19 cases. In clinical practice, there are various ways to assess the extent of pulmonary involvement in COVID-19. In general, the larger the number of the explored areas, the greater the likelihood of a significant picture of overall lung involvement.
A total of 12 areas over the chest, namely the anterosuperior, anteroinferior, laterosuperior, lateroinferior, posterosuperior, and posteroinferior lung regions on each side, showed an optimal accuracy [47]. In agreement with this method, scoring (generally from 0 to 3) each area in accordance with the most severe lung ultrasound finding gives a total gravity score (for example, when exploring six regions on each hemithorax a maximum of 36 is reached).
Clinical and experimental evidence concerning the relationships between pulmonary ultrasound signs and changes in the subpleural histology [5,7,48] allowed the formulation of a specific gravity score of COVID-19, which was initially computed in 14 areas, and published at the beginning of the pandemic [49,50]. This score is synthetized in Table 2 and in Figure 4.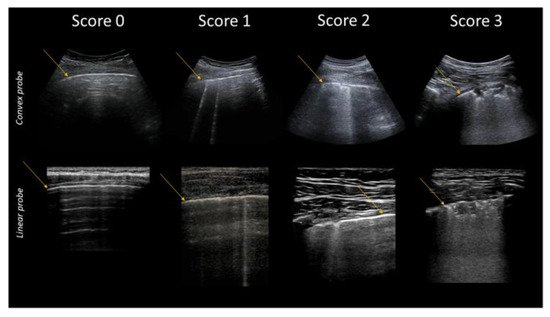

Figure 4. Classification of pathological lung ultrasound findings in COVID-19 patients. Arrows indicate the pleural line. Top: convex probe. Bottom: linear probe.
Table 2. COVID-19 Scores.
| Score | Description |
|---|---|
| 0 | Pleural line is regular. Horizontal artifacts and mirror effects are present. Normal lung. |
| 1 | Pleural line has slight alterations with sporadic vertical bright artifacts. The presence of relatively small acoustic channels due to focal interstitial thickening is speculated. |
| 2 | Pleural line has relevant alterations. Progression of subversion of peripheral air space geometry causes a predominance of vertical artifacts. Small subpleural consolidations, related to deaeration, can be present. |
| 3 | Pleural line is irregular and cobbled. Subpleural lung is denser and more disordered. White lung with or without larger consolidations may be present. Small and large consolidations are subpleural regions minimally or completely deprived of air. |
This specific methodology was subsequently validated by two studies attributing a prognostic validity to the US COVID-19 score, and proposing an evidence-based approach through a specific methodology [51,52].
Similarly, using different practical approaches, much literature data supports these original suggestions, showing both the utility of chest ultrasonography in pulmonary COVID-19 and the correlations between the diagnostic CT and ultrasound findings, whether this be at the level of a first diagnosis or as predictive tools in COVID-19 patients.
All in all, LUS represents a valuable tool in symptomatic patients with high negative predictive value for ruling out the disease.
As compared to HRCT, LUS is characterized by a very high sensitivity and specificity in detecting signs of interstitial pneumonia in COVID-19 patients (77–97% and 77–100%, respectively) [53,54,55].
From a technical point of view CT and US provide completely different assessments (CT scans lung parenchymal volumes, while in SIS, ultrasound generates a surface density map) and for this reason the diagnostic agreement between LUS and CT in terms of score was not always adequate.
However, in practical terms, LUS can be considered as an equally accurate alternative for CT in many situations where CT is not easily accessible or when molecular tests are not available. The use of lung ultrasound (LUS) as a triage tool has been proposed since the beginning of the COVID-19 pandemic [49] and subsequent studies have confirmed its role [56]. The high sensitivity of ultrasound for the superficial lesions of the lung from the interstitial stages represents its great value. Despite not showing pathognomonic COVID-19-signs, LUS is an established point-of-care tool for the evaluation of patients in the emergency department [57]. Every trained physician evaluating the admitted patients can perform an LUS to make a primary discrimination between subjects with pneumonia and subjects without pneumonia, and to monitor its pulmonary status.
Other aspects are worthy of mention. Asymptomatic carriers represent 17.9–33.3% of patients with COVID-19 [58,59] and they may contribute to the spread of the infection. The yield of screening for COVID-19 with LUS in asymptomatic patients is not known. In a retrospective study 22% of the asymptomatic patients with positive COVID-19 RT-PCR showed LUS findings. In comparison, LUS showed a positive predictive value of 100% [60].
The usefulness of LUS to predict complications in COVID-19 pneumonia has been described and sonography seems a powerful predictor of in-hospital mortality, playing a crucial role in risk stratification of patients with COVID-19.
Lung ultrasound score measured at the time of inclusion of the patients was independently associated with admission to the intensive care unit, the need for supplemental oxygen and respiratory support, and mortality. Conversely, a normal scan within 24 h of admission is indicative of a positive evolution of the pathology [61,62,63].
Moreover, LUS involvement in COVID-19 patients correlated with IL-6 levels and with the P/F ratio [64]. In hospitalized COVID-19 patients, pathological LUS was associated with venous thromboembolism [65].
Finally, using the score proposed in [50], a median value higher than 24 was associated with an almost 6-fold increase in the odds of worsening, defined as a combination of high-flow oxygen support, intensive care unit admission, or 30-day mortality as the primary end-point [51].
The worsening of pneumonia in patients with COVID-19 appears in echography with the spatial diffusion of signs of interstitial disease and consolidations. This is in accordance with the proposed grading system. On the contrary, the clinical improvement coincides with the regression of these findings (vertical artifacts, white lung, and consolidations), which leads to a downstaging of the score. Mild signs of interstitial pathology (vertical artifacts) may persist for a long time or indefinitely (see Section 7).
Beyond its use as a diagnostic and prognostic tool, LUS can be used to define optimal PEEP, guide recruitment, or monitor recruitment and should be part of the diagnostic toolset in intensive care units [66]. Ultrasound-guided recruitment is generally carried out according to the principles set out by Bouhemad et al. [67].
US signs of COVID-19 pneumonia are not specific, as they are also present to various degrees in other pathologies. Therefore, the diagnostic accuracy of pulmonary ultrasound in this pathology is strongly influenced by the pretest probability of belonging to an exposed population in a particular epidemiological context, and showing compatible symptoms.
As for any etiology of acute respiratory distress, lung ultrasound must incorporate examinations of the pleura, and of the cardiovascular system, so as to detect myocarditis, for example, and to acquire some hemodynamic data at least.
Five SARS-CoV-2 variants are known—alpha, beta, gamma, delta, and omicron. The delta variant was dominant in the summer of 2021 and the omicron variant was identified in November 2021. There are currently no data demonstrating differences in ultrasound appearance between COVID-19 variants in cases of pneumonia [68].
This entry is adapted from the peer-reviewed paper 10.3390/diagnostics12040838
This entry is offline, you can click here to edit this entry!
