Licorice (Glycyrrhiza glabra) has been largely used for thousands of years in traditional Chinese medicine. Licorice and its derived compounds possess antiallergic, antibacterial, antiviral, anti-inflammatory, and antitumor effects. G is a triterpene glycoside complex and has been shown to possess cytotoxic effects against several cancer cell lines such as colon, lung, leukemia, melanoma, and glioblastoma (GBM). GA, an aglycone of G, has been demonstrated to have pro-apoptotic effects on human hepatoma, promyelocytic leukemia, stomach cancer, Kaposi sarcoma-associated herpesvirus-infected cells, and prostate cancer cells in vitro by inducing DNA fragmentation and oxidative stress.
- Glycyrrhiza glabra-derived compounds
- glycyrrhizin (G)
- glycyrrhetinic acid (GA)
1. Introduction
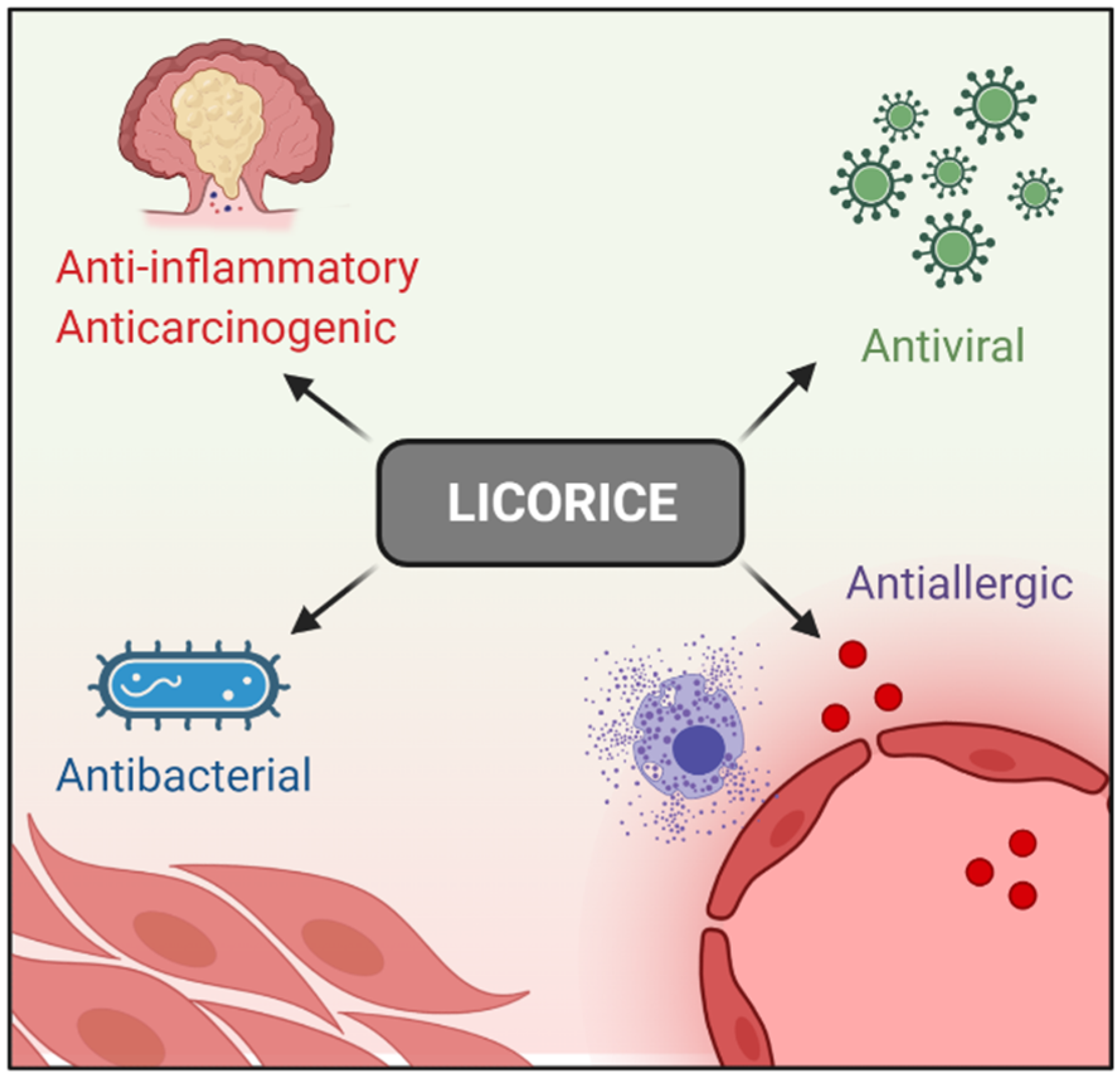 Figure 1. Licorice pharmacological properties.
Figure 1. Licorice pharmacological properties.2. G, GA, and DPG-Mediated Anti-Inflammation Regulation
3. G, GA, and DPG-Mediated Crosstalk between Inflammation and Oxidative Stress Pathways
| Model | Compound (Dose) | Mechanism | Reference |
|---|---|---|---|
| In vitro (KATO III and HL-60) | G (1 to 10 mg/mL) | Antitumor activity ↑ apoptosis | [23] |
| In vitro (HLE, KATO III, and HL-60) | G (0.1 to 1 mg/mL) | Antitumor activity ↑ apoptosis | [24] |
| In vitro (DU-145 and LNCaP) |
G (1 to 20 mM) | Antitumor activity ↑ apoptosis | [25] |
| In vitro (Caco3, HT29, and RAW 264.7) In vivo (Acute lung injury mice model) |
DPG (300 µM) DPG (3 and 8 mg/kg/day) |
↓ TNF-α, IL-1β, and IL-6, as well as HMGB1 receptors, RAGE and TLR4 | [34] |
| In vitro (neutrophils) | G (0.05, 0.5, and 5.0 µg/mL) | ↓ ROS | [38] |
| In vivo (Con A-induced hepatitis) Ex vivo (liver dendritic cells) |
G (2 mg/mouse) G (0.1 mg/mL) |
↑ IL-10 and ↓ liver inflammation | [39] |
| In vitro (U251) | GA (1, 2, 4 mM) | Anticancer effect ↓ proliferation and ↑ apoptosis possibly related to the NF-κB mediated pathway | [56] |
| In vitro (U87MG and T98G) | DPG (0.1 to 2 mM) | Anticancer effect ↓ proliferation and ↑ apoptosis. ↓ NF-κB pathway | [57] |
| In vivo (DSS-induced colitis mice model) | DPG (8 mg/kg/day) | ↓ colitis, at the earlier stages, ↓ inflammation though AMPK-COX-2-PGE. At later times ↓ iNOS and COX-2 in HMGB1-dependent manner | [42] |
| In vivo (mechanical thrombectomy rat model) | G (2, 4 and 10 mg/kg/day) | ↓ HMGB1 and its downstream inflammatory factors, and ↓ oxidative stress |
[43] |
| In vivo (Focal cerebral I/R injury rat model) | G (4 mg/kg/day) | ↓ HMGB1 and ↑ apoptosis through the blockage of the JNK and p38 | [44] |
| In vivo (Sepsis-induced acute lung injury rat model) | G (25 and 50 mg/kg/day) | ↓ inflammatory responses, oxidative stress damage, and apoptosis though ↓ NF-κB, JNK, and p38 MAPK |
[45] |
| In vivo (Acute lung injury mice model) | G (20 and 40 mg/kg/day) | ↓ LPS-induced lung injury via blocking HMGB1/TLRs/NF-κB pathway | [46] |
| In vitro (RAW 264.7 and bone marrow monocytes) | G (25 to 100 µM) | ↓ RANKL-induced osteoclastogenesis and oxidative stress through ↑ AMPK/Nrf2 and ↓ NF-κB and MAPK | [49] |
| In vivo (Parkinson rat model) | GA (50 mg/kg/day) | ↓ dopamine neuron loss and ↓ Iba-1 and GFAP ↑ antioxidant enzyme activity, ↓ lipid peroxidation, ↓ pro-inflammatory cytokines |
[51] |
| In vivo (Vascular dementia rat model) | GA (20 mg/kg/day) | ↓ release of cytochrome-c and ↑ Bcl2, and ↑ the endogenous antioxidants |
[52] |
| In vitro (HBZY-1) In vivo (sepsis-induced acute kidney injury mice model) |
GA (50 and 100 µM) GA (25 and 50 mg/kg/day) |
↓ oxidative stress via ↑ ERK signaling pathway. ↓ NF-κB | [53] |
| In vivo (myocardial ischemic injury-rat model) | GA (10 and 20 mg/kg/day) | ↓ oxidative stress and inflammatory cytokines. ↑ Nrf2 antioxidant response ↓ NF-κB activation |
[54] |
| In vitro (HEPG2) | G (5, 25 and 125 µg/mL) | ↓ H2O2-induced oxidative stress, ↑ apoptosis | [55] |
| In vitro (HT29) | GA (1, 5 and 10 µM) | ↓ TNF-α-mediated IL-8 through ↓ MAPK and the IKB/NF-κB pathway | [58] |
| In vivo (DSS-induced colitis mice model) | GA (10 and 50 mg/kg/day) | ↓ colitis, ↓ inflammation by regulating COX-2 and NF-κB | [59] |
| In vivo (rat model of ulcerative colitis) | G (40 mg/kg/day) | ↓ colitis, ↓ inflammatory injury via suppression of NF-κB, TNF-α, and ICAM-1 |
[60] |
| In vivo (TNBS-induced experimental colitis mice model) | G (10, 30 and 90 mg/kg/day) | ↓ colitis, ↓ IFN-γ, IL-12, TNF-α, and IL-17 and ↑ IL-10 | [61] |
| In vivo (DSS-induced colitis rat model) | G (2 mg rectally) | ↓ colitis, ↓ IL-1β, IL-6, TNF-α, Cxcl-2, Mcp1, and MPO | [62] |
| In vivo (TNBS-induced experimental colitis rat model) | GA (2, 10 and 50 mg/kg, rectally and 10 mg/kg/day) | ↓ colitis, ↓ serum levels of TNF-α and IL-1β, ↓ colon MPO and MDA, and ↑ SOD | [63] |
| In vivo (rat model of ulcerative colitis) | G (100 mg/kg/day) | ↓ colitis, when combined with emu synergistically ↓ of PPARγ and TNF-α | [64] |
| In vivo (TNBS-induced experimental colitis mice model) | G (50 mg/kg/day) | ↓ colitis, ↓ HMGB1 on DC/macrophage mediated Th17 proliferation | [65] |
| In vivo (indomethacin-induced small intestinal injury mice model) | GA (100 mg/kg/day) | ↓ TNF-α, IL-1β, and IL-6, ↑ indomethacin-induced small intestinal damage | [66] |
| In vivo (DSS-induced colitis mice model) | G (100 mg/kg/day) | ↓ colitis, regulated the phosphorylation of transcription factors such as NF-κB p65 and IκB α | [67] |
| In vivo (DSS-induced colitis mice model) | DPG (8 mg/kg/day) | ↑ mucosal healing by ↓ CXCL1, CXCL3, CXCL5, PTGS2, IL-1β, IL-6, CCL12, CCL7; ↑ wound healing genes COL3A1, MMP9, VTN, PLAUR, SERPINE, CSF3, FGF2, FGF7, PLAT, TIMP1 and ↑ extracellular matrix remodeling genes, VTN, and PLAUR |
[68] |
This entry is adapted from the peer-reviewed paper 10.3390/ijms23084121
References
- Deng, Q.P.; Wang, M.J.; Zeng, X.; Chen, G.G.; Huang, R.Y. Effects of glycyrrhizin in a mouse model of lung adenocarcinoma. Cell. Physiol. Biochem. 2017, 41, 1383–1392.
- Sun, X.; Zeng, H.; Wang, Q.; Yu, Q.; Wu, J.; Feng, Y.; Deng, P.; Zhang, H. Glycyrrhizin ameliorates inflammatory pain by inhibiting microglial activation-mediated inflammatory response via blockage of the HMGB1-TLR4-NF-κB pathway. Exp. Cell Res. 2018, 369, 112–119.
- Yan, T.; Wang, H.; Cao, L.; Wang, Q.; Takahashi, S.; Yagai, T.; Li, G.; Krausz, K.W.; Wang, G.; Gonzalez, F.J.; et al. Glycyrrhizin alleviates nonalcoholic steatohepatitis via modulating bile acids and meta-inflammation. Drug Metab. Dispos. 2018, 46, 1310–1319.
- Yao, L.; Sun, T. Glycyrrhizin administration ameliorates Streptococcus aureus-induced acute lung injury. Int. Immunopharmacol. 2019, 70, 504–511.
- Paudel, Y.N.; Angelopoulou, E.; Semple, B.; Piperi, C.; Othman, I.; Shaikh, M.F. Potential Neuroprotective Effect of the HMGB1 Inhibitor Glycyrrhizin in Neurological Disorders. ACS Chem. Neurosci. 2020, 11, 485–500.
- Yang, L.; Jiang, Y.; Zhang, Z.; Hou, J.; Tian, S.; Liu, Y. The anti-diabetic activity of licorice, a widely used Chinese herb. J. Ethnopharmacol. 2020, 263, 113216.
- Kwon, Y.J.; Son, D.H.; Chung, T.H.; Lee, Y.J. A Review of the Pharmacological Efficacy and Safety of Licorice Root from Corroborative Clinical Trial Findings. J. Med. Food 2020, 23, 12–20.
- Shibata, S. A drug over the millennia: Pharmacognosy, chemistry, and pharmacology of licorice. Yakugaku Zasshi 2000, 120, 849–862.
- Menegazzi, M.; Di Paola, R.; Mazzon, E.; Genovese, T.; Crisafulli, C.; Dal Bosco, M.; Zou, Z.; Suzuki, H.; Cuzzocrea, S. Glycyrrhizin attenuates the development of carrageenan-induced lung injury in mice. Pharmacol. Res. 2008, 58, 22–31.
- Gowda, P.; Patrick, S.; Joshi, S.D.; Kumawat, R.K.; Sen, E. Glycyrrhizin prevents SARS-CoV-2 S1 and Orf3a induced high mobility group box 1 (HMGB1) release and inhibits viral replication. Cytokine 2021, 142, 155496.
- Richard, S.A. Exploring the Pivotal Immunomodulatory and Anti-Inflammatory Potentials of Glycyrrhizic and Glycyrrhetinic Acids. Mediat. Inflamm. 2021, 2021, 6699560.
- Chen, K.; Yang, R.; Shen, F.-Q.; Zhu, H.-L. Advances in Pharmacological Activities and Mechanisms of Glycyrrhizic Acid. Curr. Med. Chem. 2019, 27, 6219–6243.
- Rehman, M.U.; Farooq, A.; Ali, R.; Bashir, S.; Bashir, N.; Majeed, S.; Taifa, S.; Ahmad, S.B.; Arafah, A.; Sameer, A.S.; et al. Preclinical Evidence for the Pharmacological Actions of Glycyrrhizic Acid: A Comprehensive Review. Curr. Drug Metab. 2020, 21, 436–465.
- Zhang, Q.; Ye, M. Chemical analysis of the Chinese herbal medicine Gan-Cao (licorice). J. Chromatogr. A 2009, 1216, 1954–1969.
- Abe, H.; Ohya, N.; Yamamoto, K.F.; Shibuya, T.; Arichi, S.; Odashima, S. Effects of glycyrrhizin and glycyrrhetinic acid on growth and melanogenesis in cultured B16 melanoma cells. Eur. J. Cancer Clin. Oncol. 1987, 23, 1549–1555.
- Chung, J.G.; Chang, H.L.; Lin, W.C.; Wang, H.H.; Yeh, C.C.; Hung, C.F.; Li, Y.C. Inhibition of N-acetyltransferase activity and DNA-2-aminofluorene adducts by glycyrrhizic acid in human colon tumour cells. Food Chem. Toxicol. 2000, 38, 163–172.
- Kobayashi, M.; Fujita, K.; Katakura, T.; Utsunomiya, T.; Pollard, R.B.; Suzuki, F. Inhibitory effect of glycyrrhizin on experimental pulmonary metastasis in mice inoculated with B16 melanoma. Anticancer Res. 2002, 22, 4053–4058.
- Cassileth, B.R.; Deng, G. Complementary and Alternative Therapies for Cancer. Oncologist 2004, 9, 80–89.
- Cragg, G.M.; Newman, D.J. Plants as a source of anti-cancer agents. J. Ethnopharmacol. 2005, 100, 72–79.
- Khan, R.; Khan, A.Q.; Lateef, A.; Rehman, M.U.; Tahir, M.; Ali, F.; Hamiza, O.O.; Sultana, S. Glycyrrhizic Acid Suppresses the Development of Precancerous Lesions via Regulating the Hyperproliferation, Inflammation, Angiogenesis and Apoptosis in the Colon of Wistar Rats. PLoS ONE 2013, 8, e56020.
- Huang, R.Y.; Chu, Y.L.; Jiang, Z.B.; Chen, X.M.; Zhang, X.; Zeng, X. Glycyrrhizin suppresses lung adenocarcinoma cell growth through inhibition of thromboxane synthase. Cell. Physiol. Biochem. 2014, 33, 375–388.
- Ikeda, K.; Arase, Y.; Kobayashi, M.; Saitoh, S.; Someya, T.; Hosaka, T.; Sezaki, H.; Akuta, N.; Suzuki, Y.; Suzuki, F.; et al. A long-term glycyrrhizin injection therapy reduces hepatocellular carcinogenesis rate in patients with interferon-resistant active chronic hepatitis C: A cohort study of 1249 patients. Dig. Dis. Sci. 2006, 51, 603–609.
- Hibasami, H.; Iwase, H.; Yoshioka, K.; Takahashi, H. Glycyrrhizin induces apoptosis in human stomach cancer KATO III and human promyelotic leukemia HL-60 cells. Int. J. Mol. Med. 2005, 16, 233–236.
- Hibasami, H.; Iwase, H.; Yoshioka, K.; Takahashi, H. Glycyrrhetic acid (a metabolic substance and aglycon of glycyrrhizin) induces apoptosis in human hepatoma, promyelotic leukemia and stomach cancer cells. Int. J. Mol. Med. 2006, 17, 215–219.
- Thiugnanam, S.; Xu, L.; Ramaswamy, K.; Gnanasekar, M. Glycyrrhizin induces apoptosis in prostate cancer cell lines DU-145 and LNCaP. Oncol. Rep. 2008, 20, 1387–1392.
- Sasaki, Y.F.; Kawaguchi, S.; Kamaya, A.; Ohshita, M.; Kabasawa, K.; Iwama, K.; Taniguchi, K.; Tsuda, S. The comet assay with 8 mouse organs: Results with 39 currently used food additives. Mutat. Res.—Genet. Toxicol. Environ. Mutagen. 2002, 519, 103–119.
- Isbrucker, R.A.; Burdock, G.A. Risk and safety assessment on the consumption of Licorice root (Glycyrrhiza sp.), its extract and powder as a food ingredient, with emphasis on the pharmacology and toxicology of glycyrrhizin. Regul. Toxicol. Pharmacol. 2006, 46, 167–192.
- Yang, J.; Zhou, L.; Wang, J.; Wang, C.; Davey, A.K. The disposition of diammonium glycyrrhizinate and glycyrrhetinic acid in the isolated perfused rat intestine and liver. Planta Med. 2008, 74, 1351–1356.
- Shen, C.; Zhu, J.; Song, J.; Wang, J.; Shen, B.; Yuan, H.; Li, X. Formulation of pluronic F127/TPGS mixed micelles to improve the oral absorption of glycyrrhizic acid. Drug Dev. Ind. Pharm. 2020, 46, 1100–1107.
- Yang, T.; Lan, Y.; Cao, M.; Ma, X.; Cao, A.; Sun, Y.; Yang, J.; Li, L.; Liu, Y. Glycyrrhetinic acid-conjugated polymeric prodrug micelles co-delivered with doxorubicin as combination therapy treatment for liver cancer. Colloids Surfaces B Biointerfaces 2019, 175, 106–115.
- Sui, X.; Wei, W.; Yang, L.; Zu, Y.; Zhao, C.; Zhang, L.; Yang, F.; Zhang, Z. Preparation, characterization and in vivo assessment of the bioavailability of glycyrrhizic acid microparticles by supercritical anti-solvent process. Int. J. Pharm. 2012, 423, 471–479.
- Andersen, F.A. Final report on the safety assessment of glycyrrhetinic acid, potassium glycyrrhetinate, disodium succinoyl glycyrrhetinate, glyceryl glycyrrhetinate, glycyrrhetinyl stearate, stearyl glycyrrhetinate, glycyrrhizic acid, ammonium glycyrrhizate, dipotassium glycyrrhizate, disodium glycyrrhizate, trisodium glycyrrhizate, methyl glycyrrhizate, and potassium glycyrrhizinate. Int. J. Toxicol. 2007, 26, 79–112.
- Shim, J.Y.; Bin Yim, S.; Chung, J.H.; Hong, K.S. Antiplaque and antigingivitis effects of a mouthrinse containing cetylpyridinium chloride, triclosan and dipotassium glycyrrhizinate. J. Periodontal Implant. Sci. 2012, 42, 33–38.
- Vitali, R.; Palone, F.; Cucchiara, S.; Negroni, A.; Cavone, L.; Costanzo, M.; Aloi, M.; Dilillo, A.; Stronati, L. Dipotassium Glycyrrhizate Inhibits HMGB1-Dependent Inflammation and Ameliorates Colitis in Mice. PLoS ONE 2013, 8, e66527.
- Chen, J.; Li, L.F.; Hu, X.R.; Wei, F.; Ma, S. Network Pharmacology-Based Strategy for Elucidating the Molecular Basis Forthe Pharmacologic Effects of Licorice (Glycyrrhiza spp.). Front. Pharmacol. 2021, 12, 872.
- Kotas, M.E.; Medzhitov, R. Homeostasis, Inflammation, and Disease Susceptibility. Cell 2015, 160, 816–827.
- Hunter, P. The inflammation theory of disease. EMBO Rep. 2012, 13, 968–970.
- Akamatsu, H.; Komura, J.; Asada, Y.; Niwa, Y. Mechanism of anti-inflammatory action of glycyrrhizin: Effect on neutrophil functions including reactive oxygen species generation. Planta Med. 1991, 57, 119–121.
- Abe, M.; Akbar, F.; Hasebe, A.; Horiike, N.; Onji, M. Glycyrrhizin enhances interleukin-10 production by liver dendritic cells in mice with hepatitis. J. Gastroenterol. 2003, 38, 962–967.
- van der Pol, A.; van Gilst, W.H.; Voors, A.A.; van der Meer, P. Treating oxidative stress in heart failure: Past, present and future. Eur. J. Heart Fail. 2019, 21, 425–435.
- Pisoschi, A.M.; Pop, A.; Iordache, F.; Stanca, L.; Predoi, G.; Serban, A.I. Oxidative stress mitigation by antioxidants-An overview on their chemistry and influences on health status. Eur. J. Med. Chem. 2021, 209, 112891.
- Vitali, R.; Palone, F.; Pierdomenico, M.; Negroni, A.; Cucchiara, S.; Aloi, M.; Oliva, S.; Stronati, L. Dipotassium glycyrrhizate via HMGB1 or AMPK signaling suppresses oxidative stress during intestinal inflammation. Biochem. Pharmacol. 2015, 97, 292–299.
- Mu, S.W.; Dang, Y.; Fan, Y.C.; Zhang, H.; Zhang, J.H.; Wang, W.; Sen Wang, S.; Gu, J.J. Effect of HMGB1 and RAGE on brain injury and the protective mechanism of glycyrrhizin in intracranial-sinus occlusion followed by mechanical thrombectomy recanalization. Int. J. Mol. Med. 2019, 44, 813–822.
- Gong, G.; Xiang, L.; Yuan, L.; Hu, L.; Wu, W.; Cai, L.; Yin, L.; Dong, H. Protective effect of glycyrrhizin, a direct HMGB1 inhibitor, on focal cerebral ischemia/reperfusion-induced inflammation, oxidative stress, and apoptosis in rats. PLoS ONE 2014, 9, e89450.
- Zhao, H.; Zhao, M.; Wang, Y.; Li, F.; Zhang, Z. Glycyrrhizic Acid Prevents Sepsis-Induced Acute Lung Injury and Mortality in Rats. J. Histochem. Cytochem. 2016, 64, 125–137.
- Ge, X.; Meng, X.; Fei, D.; Kang, K.; Wang, Q.; Zhao, M. Lycorine attenuates lipopolysaccharide-induced acute lung injury through the HMGB1/TLRs/NF-κB pathway. 3 Biotech 2020, 10, 369.
- Zhao, F.Q.; Wang, G.F.; Xu, D.; Zhang, H.Y.; Cui, Y.L.; Wang, Q.S. Glycyrrhizin mediated liver-targeted alginate nanogels delivers quercetin to relieve acute liver failure. Int. J. Biol. Macromol. 2021, 168, 93–104.
- Emara, N.A.; Mahmoud, M.F.; El Fayoumi, H.M.; Mahmoud, A.A.A. The renoprotective effect of glycyrrhizic acid in insulin-resistant rats exposed to aluminum involves the inhibition of TLR4/NF-κB signaling pathway. Naunyn Schmiedebergs Arch. Pharmacol. 2021, 394, 863–872.
- Li, Z.; Chen, C.; Zhu, X.; Li, Y.; Yu, R.; Xu, W. Glycyrrhizin Suppresses RANKL-Induced Osteoclastogenesis and Oxidative Stress Through Inhibiting NF-κB and MAPK and Activating AMPK/Nrf2. Calcif. Tissue Int. 2018, 103, 324–337.
- Ali, N.M.; Mahmoud, A.A.A.; Mahmoud, M.F.; El Fayoumi, H.M. Glycyrrhizic acid and silymarin alleviate the neurotoxic effects of aluminum in rats challenged with fructose-induced insulin resistance: Possible role of toll-like receptor 4 pathway. Drug Chem. Toxicol. 2019, 42, 210–219.
- Ojha, S.; Javed, H.; Azimullah, S.; Abul Khair, S.B.; Haque, M.E. Glycyrrhizic acid Attenuates Neuroinflammation and Oxidative Stress in Rotenone Model of Parkinson’s Disease. Neurotox. Res. 2016, 29, 275–287.
- Sathyamoorthy, Y.; Kaliappan, K.; Nambi, P.; Radhakrishnan, R. Glycyrrhizic acid renders robust neuroprotection in rodent model of vascular dementia by controlling oxidative stress and curtailing cytochrome-c release. Nutr. Neurosci. 2020, 23, 955–970.
- Zhao, H.; Liu, Z.; Shen, H.; Jin, S.; Zhang, S. Glycyrrhizic acid pretreatment prevents sepsis-induced acute kidney injury via suppressing inflammation, apoptosis and oxidative stress. Eur. J. Pharmacol. 2016, 781, 92–99.
- Xu, C.; Liang, C.; Sun, W.; Chen, J.; Chen, X. Glycyrrhizic acid ameliorates myocardial ischemic injury by the regulation of inflammation and oxidative state. Drug Des. Devel. Ther. 2018, 12, 1311–1319.
- Su, M.; Yu, T.; Zhang, H.; Wu, Y.; Wang, X.; Li, G. The antiapoptosis effect of glycyrrhizate on HepG2 cells induced by hydrogen peroxide. Oxid. Med. Cell. Longev. 2016, 2016, 6849758.
- Li, S.; Zhu, J.H.; Cao, L.P.; Sun, Q.; Liu, H.D.; De Li, W.; Li, J.S.; Hang, C.H. Growth inhibitory in vitro effects of glycyrrhizic acid in U251 glioblastoma cell line. Neurol. Sci. 2014, 35, 1115–1120.
- Bonafé, G.A.; dos Santos, J.S.; Ziegler, J.V.; Umezawa, K.; Ribeiro, M.L.; Rocha, T.; Ortega, M.M. Growth inhibitory effects of dipotassium glycyrrhizinate in glioblastoma cell lines by targeting microRNAs through the NF-κB signaling pathway. Front. Cell. Neurosci. 2019, 13, 216.
- Kang, O.H.; Kim, J.A.; Choi, Y.A.; Park, H.J.; Kim, D.K.; An, Y.H.; Choi, S.C.; Yun, K.J.; Nah, Y.H.; Cai, X.F.; et al. Inhibition of interleukin-8 production in the human colonic epithelial cell line HT-29 by 18 beta-glycyrrhetinic acid. Int. J. Mol. Med. 2005, 15, 981–985.
- Jeon, Y.D.; Kang, S.H.; Bang, K.S.; Chang, Y.N.; Lee, J.H.; Jin, J.S. Glycyrrhetic acid ameliorates dextran sulfate sodium-induced ulcerative colitis in vivo. Molecules 2016, 21, 523.
- Yuan, H.; Ji, W.S.; Wu, K.X.; Jiao, J.X.; Sun, L.H.; Feng, Y.T. Anti-inflammatory effect of Diammonium Glycyrrhizinate in a rat model of ulcerative colitis. World J. Gastroenterol. 2006, 12, 4578–4581.
- Sun, Y.; Cai, T.T.; Shen, Y.; Bin Zhou, X.; Chen, T.; Xu, Q. Si-Ni-San, a traditional Chinese prescription, and its active ingredient glycyrrhizin ameliorate experimental colitis through regulating cytokine balance. Int. Immunopharmacol. 2009, 9, 1437–1443.
- Kudo, T.; Okamura, S.; Zhang, Y.; Masuo, T.; Mori, M. Topical application of glycyrrhizin preparation ameliorates experimentally induced colitis in rats. World J. Gastroenterol. 2011, 17, 2223–2238.
- Liu, Y.; Xiang, J.; Liu, M.; Wang, S.; Lee, R.J.; Ding, H. Protective effects of glycyrrhizic acid by rectal treatment on a TNBS-induced rat colitis model. J. Pharm. Pharmacol. 2011, 63, 439–446.
- Sethuraman, S.N.; Swaminathan, S.; Nelson, S.B.; Palaninathan, P.S.; Gopalan, T.K.; Velayudham, P. Modulation of PPARγ and TNFα by emu oil and glycyrrhizin in ulcerative colitis. Inflammopharmacology 2015, 23, 47–56.
- Chen, X.; Fang, D.; Li, L.; Chen, L.; Li, Q.; Gong, F.; Fang, M. Glycyrrhizin ameliorates experimental colitis through attenuating interleukin-17-producing T cell responses via regulating antigen-presenting cells. Immunol. Res. 2017, 65, 666–680.
- Ishida, T.; Miki, I.; Tanahashi, T.; Yagi, S.; Kondo, Y.; Inoue, J.; Kawauchi, S.; Nishiumi, S.; Yoshida, M.; Maeda, H.; et al. Effect of 18β-glycyrrhetinic acid and hydroxypropyl γcyclodextrin complex on indomethacin-induced small intestinal injury in mice. Eur. J. Pharmacol. 2013, 714, 125–131.
- Jeon, Y.D.; Bang, K.S.; Shin, M.K.; Lee, J.H.; Chang, Y.N.; Jin, J.S. Regulatory effects of glycyrrhizae radix extract on DSS-induced ulcerative colitis. BMC Complement. Altern. Med. 2016, 16, 459.
- Stronati, L.; Palone, F.; Negroni, A.; Colantoni, E.; Mancuso, A.B.; Cucchiara, S.; Cesi, V.; Isoldi, S.; Vitali, R. Dipotassium glycyrrhizate improves intestinal mucosal healing by modulating extracellular matrix remodeling genes and restoring epithelial barrier functions. Front. Immunol. 2019, 10, 939.
