1. Introduction
Marginal zone (MZ) B-cells are innate-like, and possess a polyreactive B-cell receptor (BCR) and several pattern recognition receptors (PRR) [1][2]. They are known to generate low-affinity first-line antibody responses against invading pathogens such as encapsulated bacteria [3]. Unfortunately, deregulations affecting MZ B-cell populations have been reported in the context of Human Immunodeficiency Virus (HIV) and other chronic inflammatory conditions [2][4][5]. In here, the MZ B-cell ontogeny and antibody responses will only be briefly discuss, as these topics have been reviewed elsewhere and are beyond the scope [1][2][3][6]. Examining the regulatory capacities of MZ and other B-cell populations sharing similar features will be concentrated. The importance of the B-cell activating factor (BAFF) and its analog A Proliferation-Inducing Ligand (APRIL) in shaping the MZ B-cell pool and Breg profile will be discussed. The deregulation of MZ B-cell populations and development of MZ lymphomas (MZL) in the context of HIV and other inflammatory diseases will also be addressed. Lastly, the possible therapeutic avenues that could be deployed to restore MZ B-cell immune competence will also be talked about.
2. Ontogeny of MZ B-Cells
The first B-cell progenitors can be found as early as 7 weeks post conception in the fetal liver
[7]. However, the B lymphoid progenitor compartment differs between fetal and postnatal life, and herein, only postnatal ontogeny will be briefly concerned , as these topics are beyond the scope of this article and have been thoroughly reviewed elsewhere
[7]. As depicted in
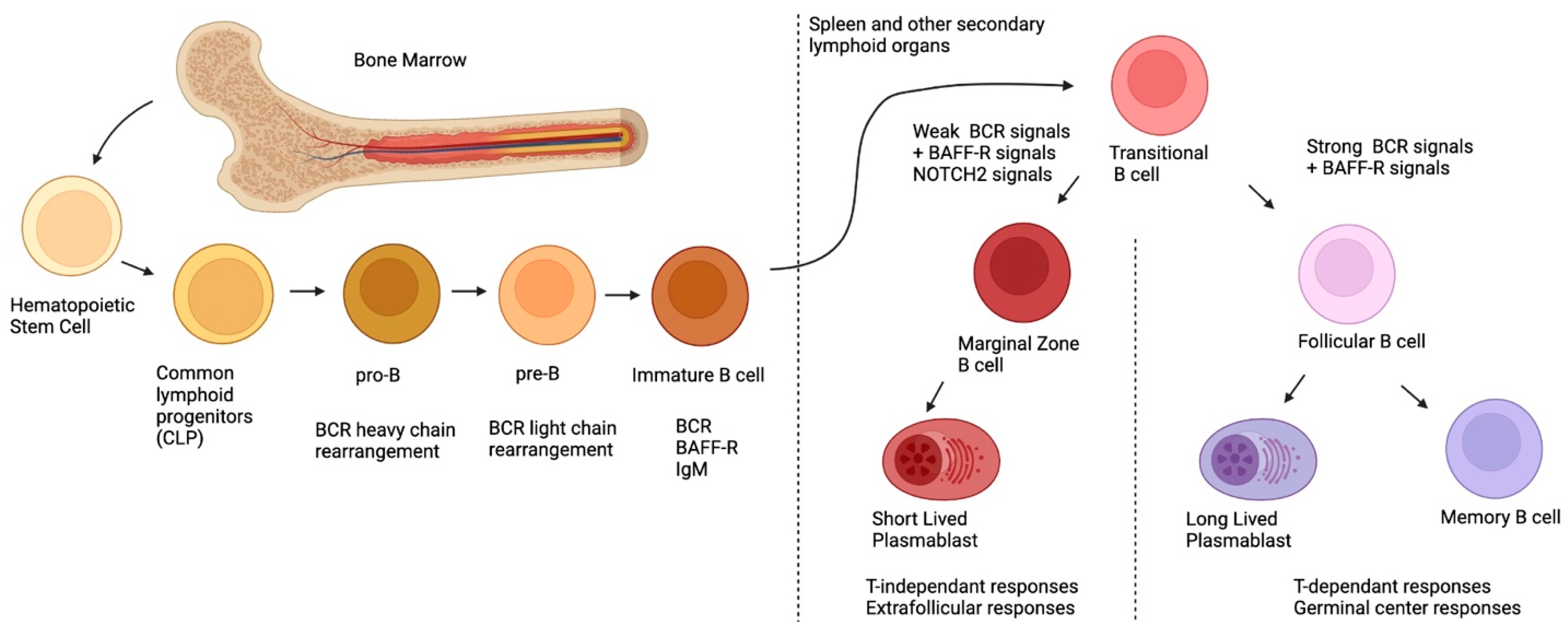
Figure 1, post-natal B-cell development originates in the bone marrow, first, with pluripotent hematopoietic stem cells and their differentiation into common lymphoid progenitors (CLP)
[8]. During their development, B-cells will undergo rearrangement of their BCR heavy chains (during the pro-B stage) and their light chains (during the pre-B stage) via the action of the Recombination-activating genes 1 and 2 (
RAG1,
RAG2)
[9]. Overall, during these recombination steps, positive and negative selection ensures that the new BCR is functional, yet not autoreactive
[10]. After these selection processes, immature B-cells, which express the newly rearranged BCR of the IgM isotype on their surface, exit the bone marrow and migrate to the spleen or other secondary lymphoid organs, where they complete their maturation and differentiation
[10]. At this point, the immature B-cell is called transitional immature (TI); it will follow maturation steps comprising stages TI-1 to TI-2 and TI-3, and will commit to either the follicular (FO) or the MZ B-cell fates, depending on the signals it receives (see below)
[11][12][13]. Although this generalized sequence of events appears to be similar for the murine and human systems, several distinctions prevail
[14]. One major difference is the fact that in the murine system, MZ B-cells are believed to arise mainly from TI-2 progenitors, which complete their maturation in the spleen, where they appear to be restricted in contrast to those observed in the human system where MZ B-cells recirculate
[15]. Interestingly, recent studies in humans have demonstrated the existence of bone-marrow-derived TI-2 IgM
lo and IgM
hi progenitors, the latter of which share transcriptional features with MZ B-cells, express α4β7 and migrate to the gut-associated lymphoid tissue (GALT)
[13]. This suggests that the GALT may be an important site for human MZ B-cell differentiation
[14].
Figure 1. Ontogeny of postnatal B-cells.
FO and MZ B-cells are known as conventional B-cells or B2 cells
[16]. Another subpopulation with innate-like properties has been identified in mice, and its cells are dubbed B1 cells. B1 and B2 cells differ in their ontogeny, as B1 cells come from a distinct lineage in the fetal liver and B2 cells originate from the bone marrow; moreover, B1 and B2 cells differ in their location, as B1 cells are most commonly found in the pleural cavity, unlike B2 cells
[16][17]. Despite the fact that B1 cells are acknowledged in mice, their presence in humans, to date, remains controversial
[17].
To date, at least three signals are involved in the FO versus MZ B-cell fate:
1. via the receptor for BAFF (BAFF-R), fundamental for sending survival signals to TI B-cells and for activating the canonical nuclear factor kappa B (NF-κB) signaling path;
2. signals resulting from the engagement of the newly expressed BCR; and
3. Notch Receptor 2 (NOTCH 2) signals, the latter two being responsible for cell fate commitment
[12]. When NOTCH2 binds to its ligand, delta-like 1 (DLL1) (expressed by a variety of cells in the spleen such as endothelial cells of the red pulp venule), in the context of weak BCR signaling, the former is internalized and translocated into the nucleus; there, it binds to DNA and allows the expression of genes involved in MZ differentiation
[18]. However, strong BCR signaling will induce Bruton’s tyrosine kinase (BTK) signals, which will inhibit the NOTCH2 signaling pathway, allowing for the expression of genes involved in FO differentiation
[19]. It is important to note that the level of BCR signaling required for MZ differentiation induces the expression of a disintegrin and metalloproteinase-containing protein 10 (ADAM10); this is required for the cleavage of NOTCH2, necessary for its nuclear translocation
[20], implying that a complete absence of BCR stimulation will impede MZ B-cell differentiation. While BAFF itself is not a direct player in MZ differentiation, it can skew the TI B-cells into differentiating into MZ by upregulating NOTCH2 expression
[12][21][22].
To recapitulate, MZ B-cells originate from the bone marrow, where they will undergo BCR rearrangement. After expressing the newly arranged BCR and BAFF-R, they will migrate to the secondary lymphoid organs where they will complete their differentiation based on three signals: BAFF-R, BCR and NOTCH2. Weaker BCR signals coupled with NOTCH2 signals will dictate the differentiation towards an MZ profile, whereas strong BCR signals and a lack of NOTCH2 signaling will dictate the differentiation towards an FO profile.
In humans, MZ B-cells are usually found in the marginal zone, a strategic region surrounding germinal centers (GC). As such, MZ B-cells have been observed in the spleen and other secondary lymphoid organs such as tonsils, lymph nodes and the GALT, in areas such as in the sub-endothelial dome of the Peyer’s patches
[15][23]. Interestingly, as mentioned above, human MZs have the capacity to recirculate in blood, a trait that has not been identified in their murine counterparts. As such, in mice, MZ B-cells appear to be restricted to the splenic marginal zone, which is at the interface between the red and white pulps, and surrounding the follicular area of the spleen
[24]. This difference contributes to fueling the controversy about the MZ’s existence in humans, given that most studies on MZ B-cell biology were conducted in mice and restricted to the spleen. The spleen is one of the most irrigated organs, at any given time receiving around 5 to 10% of the total blood volume, which is huge considering its size and oxygen consumption under steady-state conditions
[25]. One of the reasons for this lies in the fact that the spleen is involved in the “screening” of the circulatory system for bloodborne antigens
[1]. Indeed, the marginal zone is placed strategically next to the blood entries in the spleen, allowing for MZ B-cells and other innate cells such as neutrophils, dendritic cells (DCs) and macrophages to act as first-line defenders, quickly responding to antigens found in the circulation
[1][2].
The B-cell composition of the marginal zone area is heterogeneous, as populations such as B1, memory and MZ B-cells transit to, or reside within, that zone; this renders the characterization of such populations difficult, as they often share several markers. In humans, MZ B-cells are characterized by their high expression levels of the atypical major histocompatibility complex (MHC) class I molecule CD1c; the surface immunoglobulin (Ig)M and the complement receptor CD21; and the low and transient expression of CD23, a C-type lectin which is also the Fc receptor for IgE and is highly expressed by FO B-cells
[1]. Interestingly, human MZ B-cells express the memory B-cell marker CD27, and their Igs present signs of somatic hypermutations (SHMs), even though these B-cells mostly produce extra-follicular T-independent Ig responses and, therefore, are not generated from typical T-dependent GC reactions, where Ig SHM and affinity maturation usually take place (discussed below)
[26]. As such, MZ B-cells are often referred to as “antigen-experienced” cells
[3][24][27]. However, there is some evidence of “memory-like” MZ B-cells that possess a more specific affinity for some bacterial antigens
[26]. The fact that MZ B-cells express CD27, together with certain differences between human and mice, makes the classification of MZ B-cells in humans controversial, where some authors consider these cells to be unswitched IgM memory B-cells
[3][24][28]; although, several key differences between unswitched IgM memory and MZ B-cells have been documented
[24]. The current tendency to track human B-cell populations—especially in blood in the context of inflammation, based on CD27 and CD21 expression levels—makes it difficult to identify innate-like populations such as MZs, as they are of low frequencies and fall into larger groups characterized in bulk. This is likely to preclude any contribution from such rarer populations. To this end, the usage of several markers should be more widely applied in order to identify such B-cell populations, whose contribution to inflammation is non negligible, as discussed further below.
3. MZ B-Cells and Their Antibody Responses
As first-line defenders, MZ B-cells possess several PRRs such as Toll-like receptors (TLRs) and C-type lectins. Given the polyreactive nature of their BCR, MZ B-cells bear a strong autoreactive potential
[6][29][30]. They are known for their quick response against bloodborne pathogens, notably towards encapsulated bacteria
[31]. Following their activation, mostly in a T-independent manner (discussed below), they differentiate into short-lived plasma cells that will mostly produce antibodies of the IgM isotype, providing a first level of defense while awaiting a more refined adaptive response from FO B-cells
[32][33][34]. To this end, MZ B-cells have the potential to capture bloodborne antigens and then migrate from the marginal zone to the follicles (in a process known as shuttling); from there, they deliver immune-complexed antigens via antibodies through Fc receptors such as CD32, or via the complement system through complement receptors such as CD21 and CD35, to follicular dendritic cells (FDCs)
[35]. This process has been found to be fundamental to the generation of GCs.
Briefly, GC reactions are sites of antigen-specific T-dependent—notably via CD40-CD40L signaling—FO B-cell differentiation and Ig affinity maturation
[36]. Overall, there are two detectable phases in GC reactions: the dark phase where B-cells, having received signals for class switch recombination (CSR), stop expressing surface Ig and change their isotype into either of IgG, IgE or IgA, in order to gain effector functions (though CSR is not restricted to GC reactions)
[37][38][39]. During this stage, B-cells (or centroblasts) proliferate and undertake SHM to increase antibody affinity for the antigen. Following the dark phase, the light phase allows for B-cells, or centrocytes, to express somatically mutated class-switched Ig on their surface with a view to being selected
[39]. Notably, this differentiation scheme is not restricted to one round. The selection process is based on Ig affinity for the antigen presented at the surface of FDC, and on signals received by follicular helper T-cells (T
fh)
[39]. B-cells with poor affinity will undergo apoptosis by neglect. This GC process is essential to assure the maturation and selection of memory B-cells and long-lived plasma cells, which guarantee the generation of high affinity antibodies endowed with refined effector potential
[35].
As mentioned earlier, while MZ B-cells do not generate such high-affinity antibody responses, Ig produced by these cells have been shown (in humans) to bear low levels of SHM
[24][40][41][42]. MZ B-cells also have the potential to undergo CSR from IgM to IgG or IgA following the binding of BAFF to the receptor transmembrane activator and calcium modulator and cytophilin ligand interactor (TACI), which is highly expressed at the surface of MZ B-cells
[43][44]. However, these antibodies are considered of low-affinity and of a polyreactive nature, in contrast to those produced through GC reactions. Nevertheless, antibodies produced by first-line populations such as MZ B-cells may be relevant in circumstances of microbial control and mucosal homeostasis, as will be discussed.
Interestingly, MZ B-cells have also been shown to migrate to T-cell zones of secondary lymphoid organs and activate CD4
+ T-cells
[45]. Additionally, MZ B-cells are able to present antigens to invariant natural killer T-cells (iNKT), a type of NKT-cell with a restricted TCR repertoire that can recognize lipidic molecules in the context of atypical MHC class I-like molecules of the CD1 family, widely expressed by MZ B-cells
[46][47]. These MZ: iNKT cellular interactions confer activation, notably via the CD40-CD40L pathway
[47].
4. MZ B-Cell Populations and Their Regulatory Potential
Bregs are involved in the maintenance of tolerance and homeostasis of the immune system. Bregs were originally defined as IL-10 producing B-cells (or B10) in mice
[48]. Many groups have since identified different murine Breg subsets that possess anti-inflammatory suppressive mechanisms, mostly mediated by IL-10, such as T2-MZP-B-cells, MZ B-cells and B1a B-cells, amongst others (see
Table 1). As in mice, human Breg subsets have been mostly identified based on their IL-10 production capacities
[48]. Although IL-10 is an important regulatory cytokine, its production alone is not sufficient to qualify a B-cell population as Breg, since several human B-cell populations are capable of IL-10 production in the context of inflammation and upon stimulation
[5][49]. However, in these populations, IL-10 production does not persist in time; therefore, these populations could be falsely identified as true Bregs. Unlike the expression of
Forkhead Box P3 (FoxP3), which is a shared feature of regulatory T-cell populations, there is no single marker reported to identify Breg populations to date
[50]. As such, several immunoregulatory markers—such as IL-10, Programmed Death Ligand 1 (PD-L1), CD39 or CD73—have been associated with Breg potential and can help identify true Breg populations (see
Table 1). Notably, the overlap of several markers used by different groups could imply that different Breg populations might in fact be more similar than expected.
Table 1. Characteristics of several Breg populations in mice and humans.
| Species |
Population |
Phenotype |
Mechanism of Suppression |
References |
| Mouse |
B10 |
CD19 + CD5 + CD1dhi |
IL-10 |
[51][52] |
| |
MZ B-cells |
IgMhi IgDlo CD21hi CD23-CD1dhi |
IL-10 |
[53] |
| |
T2-MZP |
B220 + CD21hi CD1dhi IgMhi CD23+ |
IL-10 |
[54] |
| |
B1a |
CD90-CD5+ |
IL-10 |
[55] |
| |
Plasma cells |
CD19 + CD138 + IgM+ |
IL10, IL-35 |
[56] |
| |
Plasmablasts |
CD138 + CD44hi |
IL-10 |
[57] |
| |
Tim-1 + B-cells |
CD19 + Tim-1+ |
IL-10 |
[58] |
| |
IL-35-Bregs |
CD5 + CD1dhi FcγIibhi |
IL-35 |
[59] |
| |
GITRL + B-cells |
- |
GITRL |
[60] |
| |
Killer B-cells |
CD19 + CD5 + FasL+ |
FasL, TGF-β |
[61][62] |
| |
PD-L1hi B-cells |
CD19 + PD-L1hi |
PD-L1 |
[63] |
| |
- |
B220 + CD39 + CD73+ |
ADO, CD39 + CD73 + Extracellular vesicules |
[64][65] |
| |
GIFT-15 B-cells |
B220 + CD21 + CD22 + CD23 + CD24 + CD1d + CD138 + IgM + IgD+ |
IL-10 |
[66] |
| Human |
MZp |
CD19 + CD1c + CD21lo IgMhi CD27 + CD10+ |
CD83, PD-L1, IL-10 |
[5][67] |
| |
Transitional B-cells |
CD19 + CD24hi CD38hi |
IL-10 |
[68] |
| |
Memory B-cells |
CD19 + CD24hi CD27+ |
IL-10 |
[69] |
| |
Br1 |
CD25hi CD71hi CD73lo |
IL-10 |
[70] |
| |
TIM1 + B-cells |
CD19 + TIM1+ |
IL-10 |
[71] |
| |
Plasmablast |
CD19lo CD27hi CD38hi |
IL-10 |
[72][73] |
| |
IgA + B-cells |
CD19 + IgA+ |
IL-10, PD-L1 |
[74] |
| |
Exhausted B-cells |
CD19 + CD95+ |
CD95 |
[75] |
| |
Killer B-cells |
CD19 + CD38 + IgM + FasL+ |
FasL |
[76] |
| |
PD-L1 B-cells |
CD19 + PD-L1+ |
PD-L1 |
[62] |
| |
CD39high |
CD19 + CD39highCD73+ |
ADO |
[77] |
| |
iBreg |
- |
TGF-β, IDO |
[78] |
As mentioned earlier, the B-cell population sharing characteristics of both MZ and TI B-cells have been characterized, which was termed “precursor-like MZ B-cells” (MZp) and which bears a CD19
+IgM
highCD27
+CD1c
+CD21
lowCD10
+ phenotype
[4][5][79]. Some recent research have shown that MZps possess strong regulatory potential due to the Breg molecules that they express (see
Table 2). Indeed, besides their strong ex vivo IL-10 expression profile, MZps highly express the nuclear receptors (NR)4A1, NR4A2 and NR4A3, as well as the immunoregulatory molecule CD83 (see below)
[5][67]. Furthermore, MZps also express the ectonucleotidases CD39 and CD73, as well as several molecules associated with Breg functions, such as Transform Growth Factor Beta (TGF-β), IL-35, TLR10, Human Leukocyte Antigen G (HLA-G) and PD-L1
[67]. Strikingly, it can be found that the Breg function of MZp was directly linked with signals involving CD83, and more recently with the PD-1/PD-L1 signaling path, as discussed below
[67].
Table 2. The regulatory molecules expressed by human blood and tonsillar MZps.
| mRNA Expression |
Confirmed Protein Expression |
| NR4A1, NR4A2, NR4A3, CD83 CD39, CD73, TGF-β, IL-10, PD-L1, IL-10R, IL-27β, IL-12 p35, HLA-G |
NR4A1, NR4A3, CD83, CD39, CD73, PD-L1, IL-10 |
4.1. Importance of NR4As
The NR4As are a family of orphan nuclear receptors, meaning that their endogenous ligand is unknown. There are three known transcription factors in this family: NR4A1 (or Nur77), NR4A2 (or Nurr1) and NR4A3 (or NOR-1), all of which possess a certain degree of homology and redundant functions
[80]. Normally, a nuclear receptor must bind to its ligand in order to undergo a conformational change that allows for their DNA binding and subsequent gene transcription. However, it has been shown that the transcription factors of the NR4A family may not need such a ligand, since their natural conformation is constitutively active
[80].
Members of the NR4A family are known for their regulatory, anti-inflammatory and pro-apoptotic actions. As a matter of fact, expression of all three known members of the NR4A family is essential for the maintenance of FoxP3 expression by Tregs; moreover, their deficiency converts Treg precursors into autoreactive T-cells, possibly due to the nature of Treg selection
[81][82]. The expression of NR4As is quickly upregulated (they are “early induced genes”) following several stimulatory engagements (such as after BCR and TCR stimulation, and even TLR signaling) to control unhindered immune responses, notably in the absence of co-stimulation. Moreover, they participate in the contraction of the immunological response by inducing clonally expanded lymphocytes to undergo apoptosis
[83][84]. NR4As have been shown to be upregulated in exhausted T-cells in the context of cancer and chronic infection in mice, suggesting a contribution to the control of immune responses in the context of prolonged and/or excessive immune activation
[85][86]. The NR4As are also important for monocyte differentiation, since NR4A1 is essential for the differentiation of intermediate and non-classical monocyte subsets, and monocyte derived dendritic cells (MoDC) are absent in NR4A3 knock-out (KO) mice
[87][88][89][90]. Lastly, the NR4As are involved in the expression of immune checkpoint molecules such as PD-L1, further illustrating the immune regulatory function of these molecules
[91]. Importantly, NR4As are a part of the cyclic AMP (cAMP) response elements (CREs), the expression of which is modulated by the cAMP binding protein (CREB)
[92]. The CREB is involved in the expression of several immunoregulatory proteins and anti-inflammatory molecules such as IL-10, and it is activated by the accumulation of cAMP in the cytosol
[93].
One of the molecules whose expression is directly controlled by the NR4A family is the immunoregulatory protein CD83
[94]. Accordingly, it can be shown that MZp express high levels of CD83 ex vivo, and their Breg function is related to this molecule, as the administration of a CD83 blocking antibody impedes MZp control of CD4
+ T-cell proliferation in vitro
[67]. CD83 is a protein of the immunoglobulin-like superfamily, whose ligand is unknown. It can be found in a membrane-bound or a soluble manner, both of which seem to play different roles in immunity, with soluble CD83 (sCD83) being involved in immunoregulatory roles
[95][96][97]. It has been suggested that, similarly to other B7 family members, it can interact with other CD83 molecules in a homotypic manner, a feat that was demonstrated in DCs
[98][99][100]. CD83’s expression and role has been shown in a wide variety of regulatory cell populations. Indeed, it has been shown that sCD83 inhibits monocyte differentiation into DC, DC maturation, and DC-mediated T-cell activation
[97][98][101]. Tolerogenic DCs have also been shown to express CD83 in order to maintain mucosal homeostasis and the self- versus non-self-immunity control
[95][100]. Furthermore, Treg generation seems to rely on sCD83 and indoleamine 2-3 dioxygenase 1 (IDO-1) production by DCs. Lastly, as is the case for NR4As, CD83 is essential for the maintenance of the Treg phenotype
[102][103].
4.2. Importance of CD39 and CD73
It has previously shown that MZps express high levels of the ectonucleotidases CD39 and CD73, molecules involved in the adenosine (ADO) pathway
[67]. As such, CD39 converts the extracellular ATP (highly inflammatory, and notably generated by cell death) into ADP and AMP, and CD73 converts the latter into ADO, an anti-inflammatory molecule
[77][104]. Thus, CD39 and CD73 expression allows for the conversion of a pro-inflammatory milieu into an anti-inflammatory one. ADO production has been shown to induce a wide variety of anti-inflammatory responses
[105]. For instance, the binding of ADO to the A2
A receptor in FO B-cells impedes GC formation, BCR signaling and TLR responses
[106]. In T-cells, ADO can promote Treg generation, which will express CD39 and CD73
[107]. Furthermore, A2
A signaling (possibly autocrine or paracrine) in Tregs increases IL-10 and TGF-β production by these cells, further nourishing the anti-inflammatory environment generated by the ADO production
[108]. As such, CD39 and CD73 expression was evaluated in a wide variety of contexts, including cancer, where these molecules and ADO have been found to contribute to the maintenance of the “cold”, anti-inflammatory tumoral microenvironment
[108]. CD39 and CD73 have previously been identified as regulatory molecules on T-cells and B-cells by different groups
[105][107]. Indeed, some Breg populations were associated with CD39 and/or CD73 expression
[77]. The binding of ADO to the A2
A receptor activates adenylate cyclase, allowing for intracellular cyclic AMP (cAMP) production and accumulation, which will then inhibit the NK-κB response and the Janus Kinase (JAK)-Signal Transducer and Activator of Transcription (STAT) pathway, important for inflammatory responses
[108]. ADO binding to the A2
A receptor has been shown to upregulate NR4A expression in monocytes
[84]. Given the importance of cAMP to CREB activation, and thus, to NR4A expression, a link between the adenosine pathway and NR4A expression is to be expected.
This entry is adapted from the peer-reviewed paper 10.3390/ijms23063372