Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Cyanobacteria, also formerly called “blue-green algae”, are photosynthetic prokaryotes with ~3500 million years of existence on the planet earth. They live in a diverse range of environments, from freshwater and marine to terrestrial ecosystems. Cyanobacteria can tolerate and live in the most extreme habitats including geothermal habitats, frozen systems, and hypersaline environments.
- cyanobacteria
- Synechosystis
- microorganisms
- photosynthesis
- biofilm
- algal bloom
- photobioreactor
- motility
- run and tumble
- phototaxis
- active fluids
- rheology of active suspensions
1. Cyanobacteria
Cyanobacteria, also formerly called “blue-green algae”, are photosynthetic prokaryotes with ~3500 million years of existence on the planet earth [1][2]. They live in a diverse range of environments, from freshwater and marine [3] to terrestrial ecosystems [4]. Cyanobacteria can tolerate and live in the most extreme habitats including geothermal habitats [5], frozen systems [6], and hypersaline environments [7].
The number of cyanobacteria species is still a matter of debate and estimated to reach 8000 [8]. According to morphological characters and molecular analyses, hitherto, 5185 species have been identified and categorized: Chroococcales, Gloeobacterales, Nostocales, Oscillatoriales, Pleurocapsales, Spirulinales, and Synechococcales (Figure 1) [9].
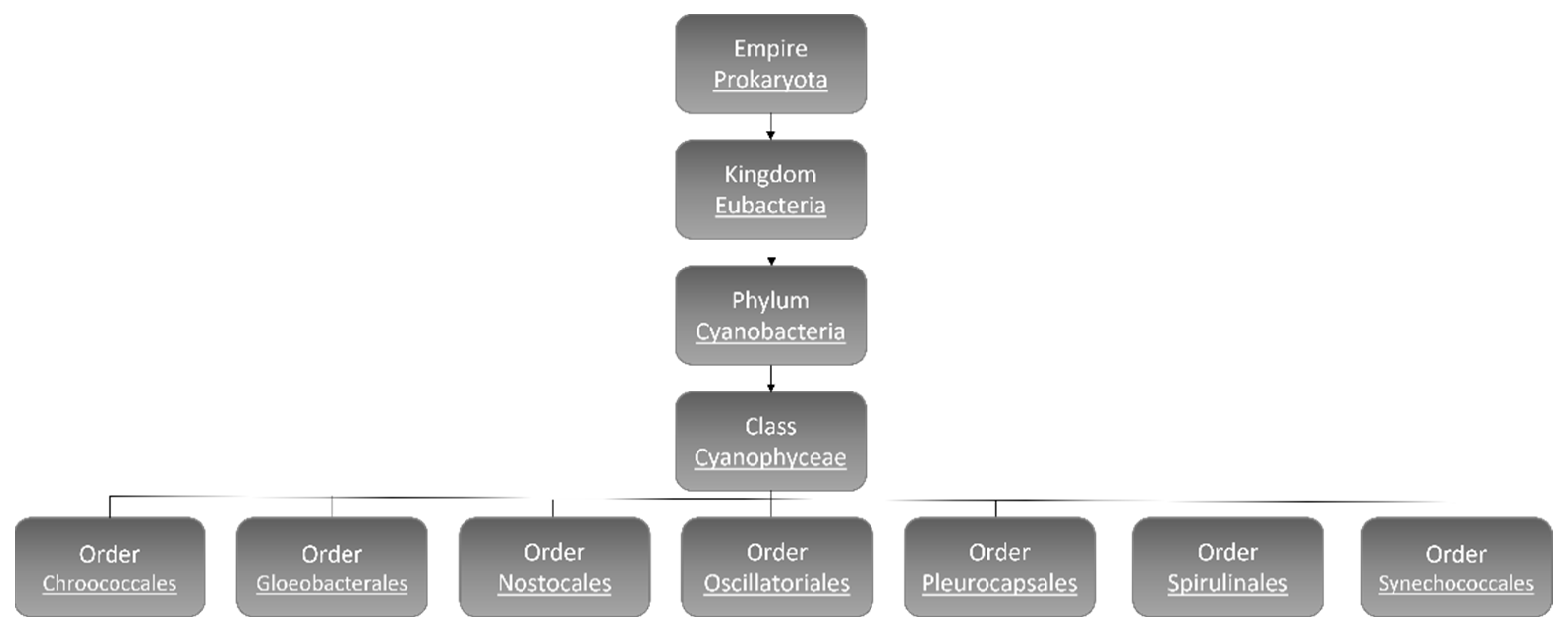
Figure 1. Cyanobacteria classification [9].
Chroococcales contain coccoid unicelled and colonial cyanobacteria inside a mucilaginous envelope. The famous species in this order is the freshwater bloom-forming species Microcystis aeruginosa [10]. Gloeobacterales are unicellular or in irregular groups rod-shaped freshwater cyanobacteria that lack thylakoids [11]. The most identified cyanobacteria are filamentous species that belong to the order of Nostocales, and some species of this order have the ability to fix nitrogen [12][13]. The highest number of benthic linear filamentous species are associated with Oscillatoriales [13] and Pleurocapsales, which can be coccoid cells or resemble filaments (pseudo-filaments), can form complex colony formations [14][15]. Spirulinales members have screw-like coiled filaments while the species categorized in Synechococcales order contain both unicellular (plus colonial) and filamentous types [15][16]. Synechococcales has more than 70 genera and is considered to be the most abundant, ecologically significant, and oldest cyanobacteria. Synechocystis sp. is a member of Synechococcales [15][16].
In aquatic habitats, unicellular cyanobacteria are considered as an important group regarding abundance, diversity, and ecological character [15]. Cyanobacteria are also responsible for the primary rise of atmospheric oxygen around 2.3 billion years ago [17] and are known as the key organisms for fixing nitrogen [18]. Recently, cyanobacteria have gained interest in producing bioenergy and valuable biocompounds. Therefore, the development of engineered cyanobacteria has attracted a great deal of attention in the last two decades [15]. One of the most popular single-cell model organisms for genetic, physiological studies of photosynthesis, and energy research is a unicellular freshwater cyanobacterium known as Synechocystis [19][20]. The entire genome of Synechocystis sp. PCC 6803, as the first phototrophic organism and the fourth organism in general, was completely sequenced in 1996 [19][21].
Cyanobacteria exist in different morphologies including, unicellular, colonial, and multicellular filamentous forms (Figure 2) [22]. Their cell size varies from less than 1 µm in diameter (Picocyanobacteria) up to 100 µm (some tropical forms in the genus Oscillatoria) [3][23][24].
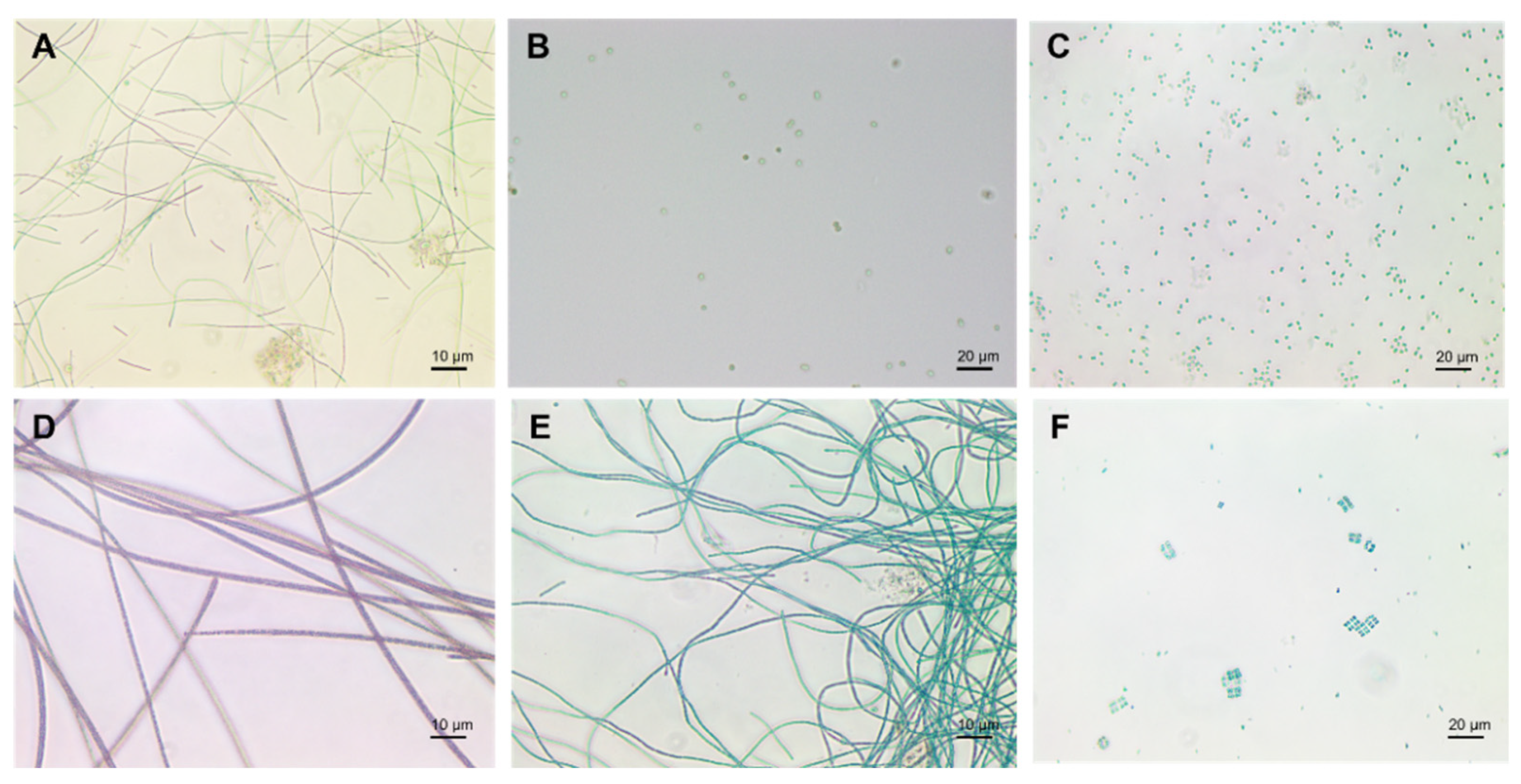
Figure 2. Cyanobacteria: (A) Plecctonema, (B) Synechocystis, (C) Microcystis, (D) Planktothrix, (E) Anabaena, (F) Merismopedia.
Unicellular cyanobacteria have spherical, ovoid, or cylindrical cells that may aggregate into irregular or regular colonies bound together by the mucous matrix (mucilage) secreted during the growth of the colony (Figure 3) [13][25]. Based on the species, the number of cells in each colony may vary from two to several thousand [15].
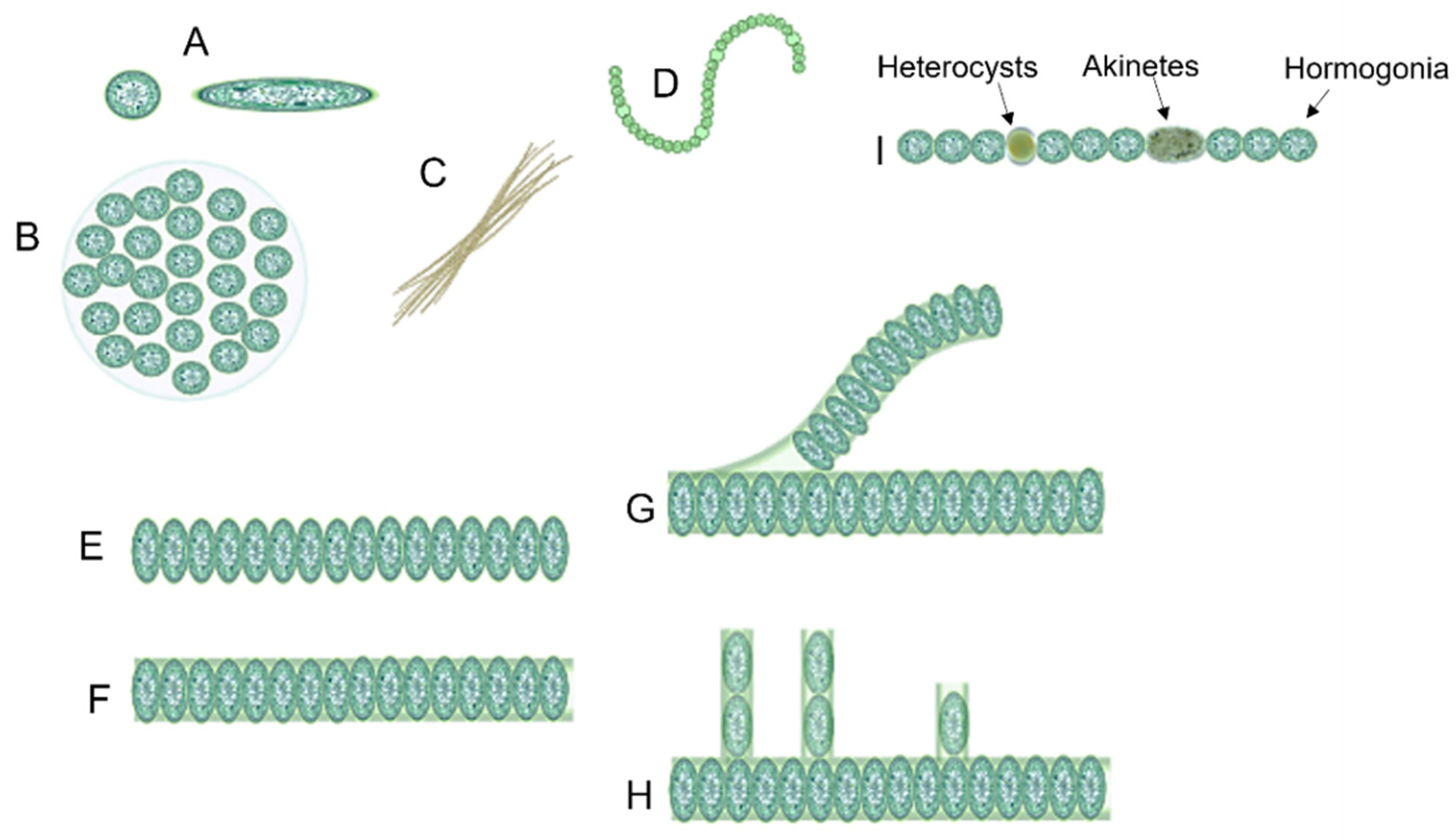
Figure 3. Different forms of cyanobacteria: (A) spherical and ovoid unicellular, (B) colonial, (C) filamentous, (D) spiral, (E) unsheathed trichome, (F) sheathed trichome, (G) false branching, (H) true branching, (I) different cell types in filamentous cyanobacteria.
After cell division, in filamentous cyanobacteria, the cells remain adhered to each other and form a chain known as “trichomes”, which can be enveloped in a mucous sheath in some taxa called filaments [13][26]. When trichomes break or fragment within a filament, false branches or “pseudobranches” are formed, which can exhibit in all cyanobacteria orders. However, in some members of Nostocales, cell division occurs perpendicular or obliquely in more than one plane leading to a true branching (Figure 3) [13][15][26].
Three different prevalent developmental cell types may differentiate from filamentous cyanobacteria vegetative cells, including hormogonia (motile cell type), heterocysts (nitrogen-fixing cell type), and akinetes (spore-like cells) (Figure 3) [27][28].
Hormogonia is a short chain of cyanobacterial filaments with gliding motility [29]. In some cyanobacteria species, hormogonia cells contain gas vacuoles to regulate buoyancy in the water column [30]. The major role of hormogonia is in the relocation and symbiotic colonization of hosts [26][30][31][32]. It was reported that the formation of hormogonia can be induced by the exchange of metabolites [33].
Heterocysts are large round-shaped cells with a thicker cell envelope in comparison with vegetative cells that are present in many multicellular cyanobacteria such as Anabaena and Nostoc and are specialized for fixing nitrogen [30][33].
Akinetes are perennating spore-like cells that can be formed under unfavorable growth conditions such as cold or drought [33]. Like heterocysts, akinetes have a thick cell wall [26]. It was hypothesized that akinetes are the evolutionary precursors of heterocysts [34].
2. Cyanobacteria’ Characteristics
2.1. Cyanobacteria’ Cell Membrane
To protect bacteria from the unpredictable and often hostile environment, their cells are enclosed by a complex multilayered structure. The structure of this layer is not similar for all bacteria and is divided into two major categories known as Gram-positive and Gram-negative, where Gram-positive bacteria are surrounded by a thick layer of peptidoglycan but lack an outer membrane. On the other hand, Gram-negative bacteria have a thin peptidoglycan cell wall, as well as an outer membrane containing lipopolysaccharide (Figure 4) [35].
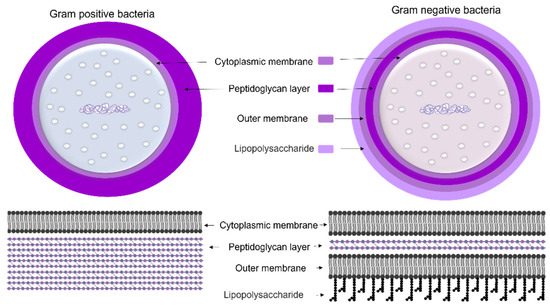
Figure 4. The cell wall composition of Gram-positive and Gram-negative bacteria.
The cell wall composition of cyanobacteria is a specific group of Gram-negative bacteria [36] with a thin layer of peptidoglycan [37] and lipopolysaccharide outer membrane [38]. However, the cyanobacteria’s peptidoglycan layer is thicker (10–700 nm) compared to most Gram-negative bacteria (2–6 nm) (Figure 5) [37]. The thin peptidoglycan layer was reported in unicellular strains such as Synechococcus (~15 nm) [39], while in filamentous strains such as Oscillatoria princeps the thickness of this layer was reported more than 700 nm [40]. Aside from protection and sensing environmental stress, transport of nutrients and metabolites into and out of the cell is one of the major roles of the cellular membrane [37].
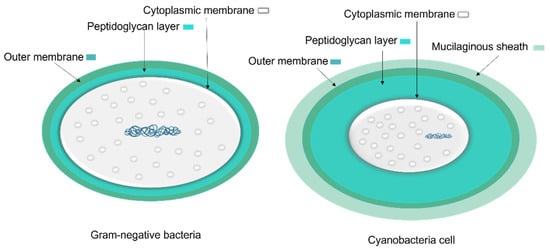
Figure 5. A comparison of general Gram-negative bacteria and cyanobacteria cell envelopes. The peptidoglycan layer in cyanobacteria is thicker than other Gram-negative bacteria and the mucilaginous sheath is present in some taxa.
Similar to Gram-negative bacteria, some cyanobacteria have extracellular non-flagellar appendages, called pili or fimbriae, which involve in motility, adhesion, biofilm formation, and uptake of DNA [41]. The pili are tube-like structures and have been characterized as type IV pili, which are protein polymers. In Synechocystis sp. PCC 6803, three different type IV pili were identified after negative staining, including thick pilus with 5–8 nm external diameter and more than 1–2 µm length; thin pilus with 2–4 nm external diameter and 0.5–1 µm length; and a bundle of pili [42][43].
2.2. Photosynthesis
Based on the occurrence, abundance, and morphological diversity, cyanobacteria are the largest group of photosynthetic prokaryotes [3], which, like higher plants, are capable of harvesting solar energy and performing photosynthesis through chlorophyll-a by fixing CO2 and generating O2 [2][44]. Cyanobacteria are responsible for a quarter of global carbon fixation, and suitable engineered photosynthetic microorganisms can increase the capacity of carbon fixation by capturing and storing CO2, and thereby stabilize or even reduce atmospheric CO2 levels [45].
In addition to chlorophyll-a (green pigment), cyanobacteria produce accessory photosynthetic blue and red pigments known as phycobilin (in particular phycocyanin (PC) and phycoerythrin (PE)), which enable them to grow under low-light conditions. They also produce carotenoids, which are known as a protective agent against photooxidative damages [22][46][47].
Quantitative determination of chlorophylls can be used as a proxy for photosynthesis, primary production, and phototaxonomic studies. However, under starvation, stress, and cell death, these pigments degrade rapidly [48]. Different cyanobacteria species, under various environmental parameters, are capable of producing various levels of these pigments [49].
Similar to eukaryotic cells, the photosynthetic apparatus of cyanobacteria is made of various units that absorb light energy and produce chemical bond energy such as reduced nicotinamide adenine dinucleotide phosphate (NADPH) and adenosine triphosphate (ATP) [50]. Chlorophyll-a contains reaction centers of photosystem I (PSI) and photosystem II (PSII). However, the major light-harvesting complex (LHC) in cyanobacteria, called the phycobilisome (PBS), is associated with the blue pigmented phycocyanin (Amax 620 nm), which absorbs photons in the orange-red part of the light spectrum, and the red-pigmented phycoerythrin (Amax 560 nm), which absorbs photons in the green-yellow part of the light spectrum, in conjunction with the PBS core of allophycocyanin (Amax 650 nm) (Figure 6) [50][51][52]. Though all PBS contain allophycocyanin and phycocyanin, some contain phycoerythrin [52].
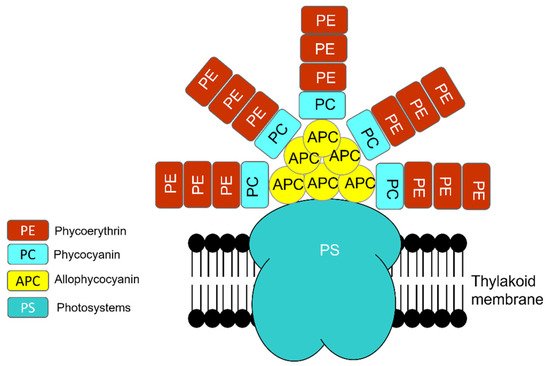
Figure 6. Phycobilisome (PBS) structure is a combination of photosystem (PS), photosynthetic reaction center that contains chlorophyll-a and PC (phycocyanin, Amax 620 nm), PE (phycoerythrin, Amax 560 nm), and APC (allophycocyanin, Amax 650 nm).
Due to the modification in light intensity and wavelength, temperature, and nutrient availability, multiple regulatory processes evolve to prevent the overexcitation of photosynthesis reaction centers, which lead to the formation of reactive oxygen species (ROS) [53][54]. The produced ROS can damage cellular components and cause cell death; therefore, it is critical to balance light harvesting in the cell [54].
2.3. Production of Secondary Metabolites
Aside from the fact that cyanobacteria are mainly oxygen-producing photosynthetic microorganisms, they are also able to develop certain mechanisms to produce a wide variety of secondary metabolites with unique structural features and biological activities in versatile ecological niches including toxins, hormones, iron chelators, antibiotics, antifungal, inflammatory and anti-inflammatory compounds, antimicrobial, antineoplastic, and cytotoxic activities [55][56][57][58][59][60][61][62]. These secondary metabolites, in general, are associated with an organism’s interaction with its environment [63] and probably can be produced to compete with other microorganisms [64]. For instance, the methanolic extract of Microcystis showed a significant anticyanobacterial activity against Anabaena BT2 and Nostoc pbr01 and antialgal activity against a green alga Bracteacoccus [58]. Moreover, some of the secondary metabolites are toxic to invertebrates, fish, birds, mammals, and humans [65]. Therefore, to protect the public from exposure to cyanotoxins guidelines values have been introduced by several countries (EU, USA, Canada, Brazil, Australia, South Africa, China, and Japan) [66].
On the other hand, the secondary metabolites can be used in the pharmaceuticals and cosmetics industry as photoprotective, antioxidants, anticancer, etc., which will be discussed more in the application section.
Till now, more than 2000 secondary metabolites have been identified, about 800 of them belonging to marine cyanobacteria. The function of most of these secondary metabolites is usually unknown [67][68].
3. Cyanobacteria in Nature and Industry
3.1. Algal Bloom
The temporal and spatial accumulation of cyanobacteria is known as “blooms” or “algal blooms” [69], which are usually beneficial for aquacultures and wild fisheries operations [70]. However, some algal blooms, known as harmful algal blooms (HABs), can have a negative impact on the aquatic ecosystem, public health, drinking water, recreation, and tourism industry and, therefore, affect the economies [69][70][71]. Climate change, global warming, and eutrophication may increase the frequency and severity of cyanobacteria blooms including the harmful ones in freshwater, estuarine, and marine ecosystems [69][72][73][74]. For instance, 20-year time series (1998–2017) for the Western Basin of Lake Erie showed cyanoHABs have become more severe (larger and longer-lasting) in recent years and accelerated after 2010 [75].
Based on the composition of cyanobacteria communities, specific types of cyanotoxins can be produced [65]. For instance, Microcystis, Planktothrix, and Anabaena are among the species that are able to produce microcystins (MCs) [76] while, Cylindrospermopsis, Aphanizomenon, Umezakia, and Anabaena are part of the group of species with the ability to excrete cylindrospermopsin (CYN) [77][78][79]. Nodularins, Anatoxins, Saxitoxins, Lyngbyatoxins, Aplysiatoxins, β-N-methylamino-L-alanine (BMAA), 2,4-diaminobutyric acid (DAB), and Lipopolysaccharides (LPS) are other cyanotoxins produced and excreted by different cyanobacteria species [79]. These toxins are released into the water when cyanobacteria cells die or lysis occurs during harmful algal blooms [80].
3.2. Usage of Cyanobacteria
Despite the negative impacts of some cyanobacteria species on the aquatic ecosystem, living organisms and human health, cyanobacteria can be beneficially used in various applications. Cyanobacteria, as the primary photosynthetic organism, can participate in carbon fixation and organic chemical production by converting CO2 into biomass and producing carbohydrates, fatty acids, and alcohols as renewable sources of biofuels [81][82].
Biofuels are considered as a renewable energy produced from grown biomass. Based on their production methods, biofuels are divided into three generations. The first-generation of biofuels are derived from edible biomass such as starch, while lignocellulosic materials from nonedible plant biomass are being used as feedstock for the second-generation. Microalgae and cyanobacteria represent the third-generation biofuels [82][83].
Some of the advantages of using cyanobacteria as a feedstock for biofuel production reside in their high growth rates and high biomass production that can be converted to biofuels or their precursors. This type of biomass production does not require arable lands used for crop growing and cultivation [81][84]. Cyanobacteria are capable to produce extensive amounts of lipids especially when the cells are under stress. Cyanobacteria can be easily manipulated genetically to convert the atmospheric carbon into biomass or desired end-products in comparison with eukaryotic microalgae [81][82].
For the mass production of microalgae on an industrial scale, very often open ponds and photobioreactors (PBRs) have been used [79][80]. Open ponds are the oldest and least expensive configurations among the different methods; however, the culture suspension in open ponds is highly susceptible to contamination with other microorganisms, difficult to control temperature fluctuations and evaporative losses, and prone to insufficient mixing and light illumination [85][86][87][88]. The biomass production in PBRs, on the other hand, is more costly from the investment point of view, but this system allows a more controllable temperature and nutrient distribution, pH, light exposure, and contamination control strategies [85][86][87][88][89]. Efficient mixing in the PBR volume can enhance the light exposure of microorganisms in suspension [88][89]. However, it should be mentioned that not all bacterial species can survive under severe shear stress caused by mechanical mixing.
One of the most innovative examples of cultivating cyanobacteria and other types of microorganisms for renewable energies and atmospheric carbon fixation is the utilization of energy-efficient bio-solar façades in green buildings. In this application, the building’s façades are covered with double-walled rectangular PBR panels [90], and the produced biomass in the façade bioreactors can be harvested on top of the building and sent to biorefinery for extraction of value-added chemical molecules [91].
Cyanobacteria can also be used as bioremediation agents to eliminate toxic wastes from contaminated sites including soil, water, wastewater, and sediments [92]. Cyanobacteria are able to degrade or detoxify many gaseous, solid, and liquid recalcitrant pollutants such as assimilate atmospheric nitrogen, remove heavy metals from aquatic ecosystems, and reduce the extra phosphate and nitrate in farmlands [93][94][95][96]. Low cost, eco-friendly nature, high efficacity, and public acceptance are the major advantages of using cyanobacteria for bioremediation [92][96][97][98].
The isolated compounds from various species of cyanobacteria of diverse habitats offer different bioactivities and structural properties with high potentials for drug development [99]. Cyanobacteria by-products express antifungal, antimicrobial, anti-inflammatory, anticoagulant, antimalarial, antiprotozoal, antiviral, antituberculosis, antitumor, immunosuppressant to anticancer, and anti-HIV activities [100][101][102][103][104][105]. For instance, the human lung cancer cell line showed apoptosis after exposure to the extract of Oscillatoria terebriformis [106], or the methanol extract of Anabaena sp. exhibited antibacterial activity against Escherichia coli MTCC-739, Staphylococcus aureus MTCC-740, Bacillus subtilis MTCC-736, Bacillus cereus MTCC-430, Bacillus pumilus MTCC-1607 [107], while the isolated compounds from Tolypothrix byssoidea EAWAG-195 revealed moderate antifungal activity against Candida albicans [108]. It has been reported that the potential extracted bioactive compounds from more than 50% of marine cyanobacteria have the ability to kill cancer cells [109]. C-phycocyanin isolated from Spirulina platensis acts as a selective inhibitor of cyclooxygenase-2 (COX-2) with hepatoprotective, anti-inflammatory, and anti-arthritic characteristics [110].
Phycocyanin and phycoerythrin pigments, isolated from cyanobacteria, can be utilized, as natural coloring agents in food, drug, and cosmetic industries to replace synthetic pigments [111]. Chewing gums, jellies, ice creams, and fermented milk products are some foodstuffs in which phycobiliproteins are used as natural food colorants [112]. The water-resistant extract of phycocyanin from Spirulina is used in cosmetic products such as eyeliner, eyeshadow, and lipsticks [57]. The market value for phycobiliprotein products was estimated to be more than USD 60 million [113]. Some unusual biomolecules, known as mycosporine-like amino acids (MAAs) and Scytonemin produced by cyanobacteria act as sunscreen agents that protect the skin cells against harmful consequences of UV radiation [114][115][116][117].
In agriculture, cyanobacteria are used as bio-fertilizer to improve soil fertility, agricultural productivity, and serve as available nitrogen sources particularly in rice cultivation in many Asian countries [118][119][120]. Employing cyanobacteria products and biomass is a more sustainable and highly effective method to improve crop production and protection due to their fertilizing, biostimulating, and biopesticide potential [121]. Free fatty acids, polysaccharides, carotenoids, and phytohormones are some cyanobacterial biologically active compounds with potential interest in agricultural practices [121]. Genetically engineered cyanobacteria can be used as multifunctional bioagents for eco-friendly and sustainable agriculture [120].
On the other hand, cyanobacteria contain many essential nutrients including proteins, amino acids, vitamins, minerals, essential fatty acids, and phytonutrients; that are being used as dietary supplements and whole food in many countries around the globe [122]. Anabaena, Nostoc, and Spirulina genera have been consumed as food for centuries [49][123].
This entry is adapted from the peer-reviewed paper 10.3390/microorganisms10040696
References
- Schopf, J.W. Microfossils of the early Archean Apex Chert: New evidence of the antiquity of life. Science 1993, 260, 640–646.
- Shestakov, S.V.; Karbysheva, E.A. The origin and evolution of cyanobacteria. Biol. Bull. Rev. 2017, 7, 259–272.
- Whitton, B.A. Diversity, ecology, and taxonomy of the cyanobacteria. In Photosynthetic Prokaryotes; Mann, N.H., Carr, N.G., Eds.; Plenum Press: New York, NY, USA, 1992; pp. 1–51.
- Lang-Yona, N.; Kunert, A.T.; Vogel, L.; Kampf, C.J.; Bellinghausen, I.; Saloga, J.; Schink, A.; Ziegler, K.; Lucas, K.; Schuppan, D.; et al. Fresh water, marine and terrestrial cyanobacteria display distinct allergen characteristics. Sci. Total Environ. 2018, 612, 767–774.
- Ward, D.M.; Castenholz, R.W.; Miller, S.R. Cyanobacteria in geothermal habitats. In Ecology of Cyanobacteria II, Their Diversity in Space and Time; Whitton, B.A., Ed.; Springer: Dordrecht, The Netherlands, 2012; pp. 39–64.
- Quesada, A.; Vincent, W.F. Cyanobacteria in the cryosphere: Snow, ice and extreme cold. In Ecology of Cyanobacteria II, Their Diversity in Space and Time; Whitton, B.A., Ed.; Springer: Dordrecht, The Netherlands, 2012; pp. 387–400.
- Oren, A. Salts and brines. In Ecology of Cyanobacteria II, Their Diversity in Space and Time; Whitton, B.A., Ed.; Springer: Dordrecht, The Netherlands, 2012; pp. 401–426.
- Guiry, M.D. How many species of algae are there? J. Phycol. 2012, 48, 1057–1063.
- Guiry, M.D.; Guiry, G.M. AlgaeBase. World-Wide Electronic Publication, National University of Ireland, Galway. 2022. Available online: https://www.algaebase.org/browse/taxonomy/#4351 (accessed on 21 March 2022).
- Komárek, J. Coccoid and colonial cyanobacteria. In Freshwater Algae of North America, Ecology and Classification; Wehr, J.D., Sheath, R.G., Eds.; Academic Press: San Diego, CA, USA, 2003; pp. 59–116.
- Guiry, M.D.; Guiry, G.M. AlgaeBase. World-Wide Electronic Publication, National University of Ireland, Galway. 2015. Available online: https://www.algaebase.org/search/species/detail/?species_id=153246 (accessed on 21 March 2022).
- Dolman, A.M.; Rücker, J.; Pick, F.R.; Fastner, J.; Rohrlack, T.; Mischke, U.; Wiedner, C. Cyanobacteria and cyanotoxins: The influence of nitrogen versus phosphorus. PLoS ONE 2012, 7, e38757.
- Vidal, L.; Ballot, A.; Azevedo, S.M.F.O.; Padisák, J.; Welker, M. Introduction to cyanobacteria. In Toxic Cyanobacteria in Water, 2nd ed.; Chorus, I., Welker, M., Eds.; CRC Press: Boca Raton, FL, USA, 2021; pp. 163–211.
- Vincent, W.F. Cyanobacteria. In Plankton of Inland Waters; Liknes, G.E., Ed.; Academic Press: San Diego, CA, USA, 2009; pp. 142–148.
- Dvořák, P.; Casamatta, D.A.; Hašler, P.; Jahodářová, E.; Norwich, A.R.; Poulíčková, A. Diversity of the cyanobacteria. In Modern Topics in the Phototrophic Prokaryotes; Hallenbeck, P., Ed.; Springer: Cham, Switzerland, 2017; pp. 3–46.
- Komárek, J.; Kaštovský, J.; Mareš, J.; Johansen, J.R. Taxonomic classification of cyanoprokaryotes (cyanobacterial genera) 2014, using a polyphasic approach. Preslia 2014, 86, 295–335.
- Holland, H.D. Volcanic gases, black smokers, and the great oxidation event. Geochim. Cosmochim. Acta 2002, 66, 3811–3826.
- Tyrrell, T. The relative influences of nitrogen and phosphorus on oceanic primary production. Nature 1999, 400, 525–531.
- Ikeuchi, M.; Tabata, S. Synechocystis sp. PCC 6803—A useful tool in the study of the genetics of cyanobacteria. Photosynth. Res. 2001, 70, 73–83.
- Lindberg, P.; Park, S.; Melis, A. Engineering a platform for photosynthetic isoprene production in cyanobacteria, using Synechocystis as the model organism. Metab. Eng. 2010, 12, 70–79.
- Kaneko, T.; Sato, S.; Kotani, H.; Tanaka, A.; Asamizu, E.; Nakamura, Y.; Miyajima, N.; Hirosawa, M.; Sugiura, M.; Sasamoto, S.; et al. Sequence analysis of the genome of the unicellular cyanobacterium Synechocystis sp. strain PCC 6803. II. sequence determination of the entire genome and assignment of potential protein-coding regions. DNA Res. 1996, 3, 109–136.
- Humbert, J.F.; Fastner, J. Ecology of cyanobacteria. In Handbook of Cyanobacterial Monitoring and Cyanotoxin Analysis; Meriluoto, J., Spoof, L., Codd, G.A., Eds.; John Wiley & Sons, Ltd.: Chichester, UK, 2017; pp. 11–18.
- Schulz-Vogt, H.N.; Angert, E.R.; Garcia-Pichel, F. Giant Bacteria. eLS 2007, 1–7.
- Jasser, I.; Callieri, C. Picocyanobacteria: The smallest cell-size cyanobacteria. In Handbook of Cyanobacterial Monitoring and Cyanotoxin Analysis; Meriluoto, J., Spoof, L., Codd, G.A., Eds.; John Wiley & Sons, Ltd.: Chichester, UK, 2017; pp. 19–27.
- Mur, L.R.; Skulberg, O.M.; Utkilen, H. Cyanobacteria in the environment. In Toxic Cyanobacteria in Water: A Guide to Their Public Health Consequences, Monitoring and Management; Chorus, I., Bartram, J., Eds.; CRC Press: Boca Raton, FL, USA, 1999; pp. 15–40.
- Uyeda, J.C.; Harmon, L.J.; Blank, C.E. A comprehensive study of cyanobacterial morphological and ecological evolutionary dynamics through deep geologic time. PLoS ONE 2016, 9, e0162539.
- Singh, S.P.; Montgomery, B.L. Determining cell shape: Adaptive regulation of cyanobacterial cellular differentiation and morphology. Trends. Microbiol. 2011, 19, 278–285.
- Dvořák, P.; Poulíčková, A.; Hasler, P.; Belli, M.; Casamatta, D.A.; Papini, A. Species concepts and speciation factors in cyanobacteria, with connection to the problems of diversity and classification. Biodivers. Conserv. 2015, 24, 739–757.
- Rippka, R.; Deruelles, J.; Waterbury, J.B.; Herdman, M.; Stanier, R.Y. Generic assignments, strain histories and properties of pure cultures of cyanobacteria. J. Gen. Microbiol. 1979, 111, 1–61.
- Meeks, J.C.; Elhai, J. Regulation of cellular differentiation in filamentous cyanobacteria in free-living and plant-associated symbiotic growth states. Microbiol. Mol. Biol. R. 2002, 66, 94–121.
- Wong, F.C.Y.; Meeks, J.C. Establishment of a functional symbiosis between the cyanobacterium Nostoc punctiforme and the bryophyte Anthoceros punctatus requires genes involved in nitrogen control and initiation of heterocyst differentiation. Microbiology 2002, 148, 315–323.
- Adams, D.G.; Duggan, P.S. Cyanobacteria–bryophyte symbioses. J. Exp. Bot. 2008, 59, 1047–1058.
- Kumar, K.; Mella-Herrera, R.A.; Golden, J.W. Cyanobacterial heterocysts. Cold Spring Harb. Perspect. Biol. 2010, 2, 1–20.
- Wolk, C.P.; Ernst, A.; Elhai, J. Heterocyst metabolism and development. In the Molecular Biology of Cyanobacteria; Bryant, D.A., Ed.; Springer: Dordrecht, The Netherlands, 1994; pp. 769–823.
- Silhavy, T.J.; Kahne, D.; Walker, S. The bacterial cell envelope. Cold Spring Harb. Perspect. Biol. 2010, 2, a000414.
- Woese, C.R. Bacterial Evolution. Microbiol. Rev. 1987, 51, 221–271.
- Hoiczyk, E.; Hansel, A. Cyanobacterial cell walls: News from an unusual prokaryotic envelope. J. Bacteriol. 2000, 182, 1191–1199.
- Durai, P.; Batool, M.; Choi, S. Structure and effects of cyanobacterial lipopolysaccharides. Mar. Drugs 2015, 13, 4217–4230.
- Samuel, A.D.T.; Petersen, J.D.; Reese, T.S. Envelope structure of Synechococcus sp. WH8113, a nonflagellated swimming cyanobacterium. BMC Microbiol. 2001, 1, 4.
- Hoiczyk, E.; Baumeister, W. Envelope structure of four gliding filamentous cyanobacteria. J. Bacteriol. 1995, 177, 2387–2395.
- Schuergers, N.; Wilde, A. Appendages of the cyanobacterial cell. Life 2015, 5, 700–715.
- Bhaya, D.; Bianco, N.R.; Bryant, D.; Grossman, A.R. Type IV pilus biogenesis and motility in the cyanobacterium Synechocystis sp. PCC6803. Mol. Microbiol. 2000, 37, 941–951.
- Yoshihara, S.; Geng, X.X.; Okamoto, S.; Yura, K.; Murata, T.; Go, M.; Ohmori, M.; Ikeuchi, M. Mutational analysis of genes involved in pilus structure, motility and transformation competency in the unicellular motile cyanobacterium Synechocystis sp. PCC 6803. Plant Cell Physiol. 2001, 42, 63–73.
- Angermayr, S.A.; Hellingwerf, K.J.; Lindblad, P.; de Mattos, M.J.T. Energy biotechnology with cyanobacteria. Curr. Opin. Biotech. 2008, 20, 257–263.
- Durall, C.; Lindblad, P. Mechanisms of carbon fixation and engineering for increased carbon fixation in cyanobacteria. Algal Res. 2015, 11, 263–270.
- Barsanti, L.; Gualtieri, P. General overview. In Algae, Anatomy, Biotechemisty and Bioteconlogy, 2nd ed.; Barsanti, L., Gualtieri, P., Eds.; CRC Press: Boca Raton, FL, USA, 2014; pp. 1–48.
- Zakar, T.; Laczko-Dobos, H.; Toth, T.N.; Gombos, Z. Carotenoids assist in cyanobacterial photosystem II assembly and function. Front. Plant Sci. 2016, 7, 295.
- Jensen, A. Chlorophylls and carotenoids. In Handbook of Phycological Methods. Physiological and Biochemical Methods; Hellebust, J.A., Craigie, J.S., Eds.; Cambridge University Press: New York, NY, USA, 1978; pp. 59–70.
- Jaiswal, A.; Koli, D.K.; Kumar, A.; Kumar, S.; Sagar, S. Pigments analysis of cyanobacterial strains. Int. J. Chem. Stud. 2018, 6, 1248–1251.
- Grossman, A.R.; Bhaya, D.; Apt, K.E.; Kehoe, D.M. Light-harvesting complexes in oxygenic photosynthesis: Diversity, control, and evolution. Annu. Rev. Genet. 1995, 29, 231–287.
- Stomp, M.; Huisman, J.; de Jongh, F.; Veraart, A.J.; Gerla, D.; Rijkeboer, M.; Ibelings, B.W.; Wollenzien, U.I.A.; Stal, L.J. Adaptive divergence in pigment composition promotes phytoplankton biodiversity. Nature 2004, 432, 5–8.
- Oliver, R.L.; Hamilton, D.P.; Brookes, J.D.; Ganf, G.G. Physiology, blooms and prediction of planktonic cyanobacteria. In Ecology of Cyanobacteria II, Their Diversity in Space and Time; Whitton, B.A., Ed.; Springer: Dordrecht, The Netherlands, 2012; pp. 155–194.
- Singh, A.K.; Elvitigala, T.; Bhattacharyya-Pakrasi, M.; Aurora, R.; Ghosh, B.; Pakrasi, H.B. Integration of carbon and nitrogen metabolism with energy production is crucial to light acclimation in the cyanobacterium Synechocystis. Plant Physiol. 2008, 148, 467–478.
- Moore, K.A.; Altus, S.; Tay, J.W.; Meehl, J.B.; Johnson, E.B.; Bortz, D.M.; Cameron, J.C. Mechanical regulation of photosynthesis in cyanobacteria. Nat. Microbiol. 2020, 5, 757–767.
- Moore, R.E. Cyclic peptides and depsipeptides from cyanobacteria: A review. J. Ind. Microbiol. 1996, 16, 134–143.
- Rawat, D.; Bhargava, S. Bioactive compounds from Nostoc species. Curr. Res. Pharm. Sci. 2011, 2, 48–54.
- Sharma, N.K.; Tiwari, S.P.; Tripathi, K.; Rai, A.K. Sustainability and cyanobacteria (blue-green algae): Facts and challenges. J. Appl. Phycol. 2011, 23, 1059–1081.
- Yadav, S.; Sinha, R.P.; Tyagi, M.B. Antimicrobial activity of some cyanobacteria. Int. J. Pharm. Pharm. Sci. 2012, 4, 631–635.
- Méjean, A.; Payraud, T.H.; Kerbrat, A.S.; Goulbic, S.; Pauillac, S.; Chinain, M.; Laurent, D. First identification of the neurotoxin homoanatoxin-a from mast of Hydrocoleum lyngbyaceum (marine cyanobacterium) possibly liked to giant calm poisoning in New Caledonia. Toxicon 2010, 56, 829–835.
- Cadel-Six, S.; Peyraud-Thomas, C.; Brient, L.; de Marsac, N.T.; Ripka, R.; Méjean, A. Different genotypes of anatoxin-producing cyanobacteria coexist in the Tarn River, France. Appl. Environ. Microbiol. 2007, 73, 7605–7614.
- Méjean, A.; Mazmouz, R.; Essadik, I.; Ploux, O. Anatoxin-a and analogs: Occurrence, biosynthesis and detection. Toxicon 2018, 149, 107.
- Méjean, A.; Mazmouz, R.; Mann, S.; Calteau, A.; Medigues, C.; Ploux, O. The genome sequence of the cyanobacterium oscillatoria sp. 6506 reveals several gene clusters responsible for the biosynthesis of toxins and secondary metabolites. J. Bacteriol. 2010, 192, 5264–5265.
- Maschek, J.A.; Baker, B.J. The chemistry of algal secondary metabolism. In Algal Chemical Ecology; Amsler, C.D., Ed.; Springer: Berlin, Germany, 2008; pp. 1–24.
- Abed, R.M.M.; Dobretsov, S.; Sudesh, K. Applications of cyanobacteria in biotechnology. J. Appl. Microbiol. 2009, 106, 1–12.
- Van de Waal, D.B.; Smith, V.H.; Declerck, S.A.J.; Stam, E.C.M.; Elser, J.J. Stoichiometric regulation of phytoplankton toxins. Ecol. Lett. 2014, 17, 736–742.
- Picardo, M.; Filatova, D.; Nunez, O.; Farré, M. Recent advances in the detection of natural toxins in freshwater environments. Trends Analyt. Chem. 2019, 112, 75–86.
- Chauvat, F.; Cassier-Chauvat, C. Genomics of cyanobacteria: New insights and lessons for shaping our future—A follow-up of volume 65: Genomics of cyanobacteria. In Advances in Botanical Research; Jacquot, J.P., Ed.; Academic Press: Cambridge, MA, USA, 2021; Volume 100, pp. 213–235.
- Filatova, D.; Jones, M.R.; Haley, J.A.; Núñez, O.; Farré, M.; Janssen, E.M.L. Cyanobacteria and their secondary metabolites in three freshwater reservoirs in the United Kingdom. Environ. Sci. Eur. 2021, 33, 29.
- Paerl, H.W.; Huisman, J. Climate change: A catalyst for global expansion of harmful cyanobacterial blooms. Environ. Microbiol. Rep. 2009, 1, 27–37.
- Hallegraeff, G.M. A review of harmful algal blooms and their apparent global increase. Phycologia 1993, 32, 79–99.
- Anderson, D.M.; Cembella, A.D.; Hallegraeff, G.M. Progress in understanding harmful algal blooms: Paradigm shifts and new technologies for research, monitoring, and management. Annu. Rev. Mar. Sci. 2012, 4, 143–176.
- Moore, S.K.; Trainer, V.L.; Mantua, N.J.; Parker, M.S.; Laws, E.A.; Backer, L.C.; Fleming, L.E. Impacts of climate variability and future climate change on harmful algal blooms and human health. Environ. Health 2008, 7, S4.
- O’Neil, J.M.; Davis, T.W.; Burford, M.A.; Gobler, C.J. The rise of harmful cyanobacteria blooms: The potential roles of eutrophication and climate change. Harmful Algae 2012, 14, 313–334.
- Wells, M.L.; Trainer, V.L.; Smayda, T.J.; Karlson, B.S.O.; Trick, C.G.; Kudela, R.M.; Ishikawa, A.; Bernard, S.; Wulff, A.; Anderson, D.M.; et al. Harmful algal blooms and climate change: Learning from the past and present to forecast the future. Harmful Algae 2015, 49, 68–93.
- Sayers, M.J.; Grimm, A.G.; Shuchman, R.A.; Bosse, K.R.; Fahnenstiel, G.L.; Ruberg, S.A.; Leshkevich, G.A. Satellite monitoring of harmful algal blooms in the Western Basin of Lake Erie: A 20-year time-series. J. Great Lakes Res. 2019, 45, 508–521.
- Sivonen, K.; Jones, G. Cyanobacterial toxins. In Encyclopedia of Microbiology; Schaechter, M., Ed.; Elsevier: Oxford, UK, 2009; pp. 290–307.
- Schembri, M.A.; Neilan, B.A.; Saint, C.P. Identification of genes implicated in toxin production in the cyanobacterium Cylindrospermopsis raciborskii. Environ. Toxicol. 2001, 16, 413–421.
- Preußel, K.; Stüken, A.; Wiedner, C.; Chorus, I.; Fastner, J. First report on cylindrospermopsin producing Aphanizomenon flos-aquae (cyanobacteria ) isolated from two German lakes. Toxicon 2006, 47, 156–162.
- Codd, G.A.; Meriluoto, J.; Metcalf, J.S. Introduction: Cyanobacteria, cyanotoxins, their human impact, and risk management. In Handbook of Cyanobacterial Monitoring and Cyanotoxin Analysis; Meriluoto, J., Spoof, L., Codd, G.A., Eds.; John Wiley & Sons, Ltd.: Chichester, UK, 2017; pp. 3–8.
- Wang, C.C.; Petty, E.E.; Smith, C.M. Rapid and efficient analysis of microcystins, nodularin, cylindrospermopsin, and anatoxin-a in drinking water by LC-MS-MS. J. AOAC Int. 2016, 99, 1565–1571.
- Sarsekeyeva, F.; Zayadan, B.K.; Usserbaeva, A.; Bedbenov, V.S.; Sinetova, M.A.; Los, D.A. Cyanofuels: Biofuels from cyanobacteria. Reality and perspectives. Photosynth. Res. 2015, 125, 329–340.
- Quintana, N.; Van der Kooy, F.; Van de Rhee, M.D.; Voshol, G.P.; Verpoorte, R. Renewable energy from cyanobacteria: Energy production optimization by metabolic pathway engineering. Appl. Microbiol. Biot. 2011, 91, 471–490.
- Alalwan, H.A.; Alminshid, A.H.; Aljaafari, H.A. Promising evolution of biofuel generations. Subject review. Renew. Energy Focus 2019, 28, 127–139.
- Barka, A.; Blecker, C. Microalgae as a potential source of single-cell proteins. Biotechnol. Agron. Soc. Environ. 2016, 20, 427–436.
- Chisti, Y. Biodiesel from microalgae. Biotechnol. Adv. 2007, 25, 294–306.
- Shen, Y.; Yuan, W.; Pei, Z.J.; Wu, Q.; Mao, E. Microalgae mass production methods. Trans. ASABE 2009, 52, 1275–1287.
- Carvalho, A.P.; Meireles, L.A.; Malcata, F.X. Microalgal reactors: A review of enclosed system designs and performances. Biotechnol. Progr. 2006, 22, 1490–1506.
- Yadala, S.; Cremaschi, S. Design and optimization of artificial cultivation units for algae production. Energy 2014, 78, 23–39.
- Pruvost, J.; Cornet, J.F.; Goetz, V.; Legrand, J. Theoretical investigation of biomass productivities achievable in solar rectangular photobioreactors for the cyanobacterium Arthrospira platensis. Biotechnol. Prog. 2012, 28, 699–714.
- Mazard, S.; Penesyan, A.; Ostrowski, M.; Paulsen, I.T.; Egan, S. Tiny microbes with a big impact: The role of cyanobacteria and their metabolites in shaping our future. Mar. Drugs 2016, 14, 97.
- IBA Hamburg—BIQ. Bio Intelligent Quotient (BIQ) House in Hamburg. Smart Material Houses. 2020. Available online: https://www.internationale-bauausstellung-hamburg.de/en/projects/the-building-exhibition-within-the-building-exhibition/smart-material-houses/biq/projekt/biq.html (accessed on 21 March 2022).
- Gupta, R.K.; Chaudhary, K.K.; Kumar, M.; Negi, A.; Rai, H. Bioremediation and cyanobacteria: An overview. BioNano Front. 2012, 9, 190–196.
- Hall, D.O.; Markov, S.A.; Watanabe, Y.; Rao, K.K. The potential applications of cyanobacterial photosynthesis for clean technologies. Photosynth. Res. 1995, 46, 159–167.
- Flores, E.; Herrero, A. Nitrogen assimilation and nitrogen control in cyanobacteria. Biochem. Soc. Trans. 2005, 33, 164–167.
- Ono, E.; Cuello, J.L. Carbon dioxide mitigation using thermophilic cyanobacteria. Biosyst. Eng. 2007, 96, 129–134.
- Roeselers, G.; van Loosdrecht, M.C.M.; Muyzer, G. Phototrophic biofilms and their potential applications. J. Appl. Phycol. 2008, 20, 227–235.
- Dubey, S.K.; Dubey, J.; Mehra, S.; Tiwari, P.; Bishwas, A.J. Potential use of cyanobacterial species in bioremediation of industrial effluents. Afr. J. Biotechnol. 2011, 10, 1125–1132.
- Vijayakumar, S. Potential applications of cyanobacteria in industrial effluents—A review. J. Bioremed. Biodegrad. 2012, 3, 2–4.
- Kumar, B.N.P.; Mahaboobi, S.; Satyam, S. Cyanobacteria: A potential natural source for drug discovery and bioremediation. J. Ind. Pollut. Control 2016, 32, 508–517.
- Dittmann, M.E. Molecular biology of peptide and polyketide biosynthesis in cyanobacteria. Appl. Microbiol. Biot. 2001, 57, 467–473.
- Simmons, T.L.; Andrianasolo, E.; Mcphail, K.; Flatt, P.; Gerwick, W.H. Marine natural products as anticancer drugs. Mol. Cancer Ther. 2005, 4, 333–343.
- Volk, R. Screening of microalgae for species excreting norharmane, a manifold biologically active indole alkaloid. Microbiol. Res. 2008, 163, 307–313.
- Wase, N.V.; Wright, P.C. Systems biology of cyanobacterial secondary metabolite production and its role in drug discovery. Expert Opin. Drug Dis. 2008, 3, 903–930.
- Mayer, A.M.S.; Rodríguez, A.D.; Berlinck, R.G.S.; Hamann, M.T. Marine pharmacology in 2005–2006: Marine compounds with anthelmintic, antibacterial, anticoagulant, antifungal, anti-inflammatory, antimalarial, antiprotozoal, antituberculosis, and antiviral activities; affecting the cardiovascular, immune and nervous systems, and other miscellaneous mechanisms of action. Biochim. Biophys. Acta 2009, 1790, 283–308.
- Vijayakumar, S.; Menakha, M. Pharmaceutical applications of cyanobacteria—A review. J. Acute Med. 2015, 5, 15–23.
- Mukund, S.; Sivasubramanian, V. Anticancer activity of Oscillatoria terebriformis cyanobacteria in human lung cancer cell line A549. Int. J. Appl. Biol. Pharm. 2014, 5, 34–45.
- Chauhan, A.; Chauhan, G.; Gupta, P.C.; Goyal, P.; Kaushik, P. In vitro antibacterial evaluation of Anabaena sp. against several clinically significant microflora and HPTLC analysis of its active crude extracts. Indian J. Pharmacol. 2010, 42, 104–106.
- Jaki, B.; Zerbe, O.; Heilmann, J.; Sticher, O. Two novel cyclic peptides with antifungal activity from the cyanobacterium Tolypothrix byssoidea (EAWAG 195). J. Nat. Prod. 2001, 64, 154–158.
- Bajpai, V.K.; Shukla, S.; Kang, S.M.; Hwang, S.K.; Song, X.; Huh, Y.S.; Han, Y.K. Developments of cyanobacteria for nano-marine drugs: Relevance of nanoformulations in cancer therapies. Mar. Drugs 2018, 16, 179.
- Reddy, C.M.; Bhat, V.B.; Kiranmai, G.; Nasra Reddy, M.; Reddanna, P.; Madyastha, K.M. Selective inhibition of cyclooxygenase-2 by c-phycocyanin, a biliprotein from Spirulina platensis. Biochem. Bioph. Res. Commun. 2000, 603, 599–603.
- Cohen, Z. The chemical of Spirulina. In Spirulina platensis (Arthrospira) Physiology, Cell-Biology and Biotechnology; Vonshak, A., Ed.; Taylor and Francis Ltd.: London, UK, 1997; pp. 175–204.
- Ghosh, T.; Paliwal, C.; Maurya, R.; Mishra, S. Microalgal rainbow colours for nutraceutical and pharmaceutical applications. In Plant Biology and Biotechnology; Volume I: Plant Diversity, Organization, Function and Improvement; Bahadur, B., Rajam, M.V., Sahijram, L., Krishnamurthy, K.V., Eds.; Springer: New Delhi, India, 2015; pp. 777–791.
- Borowitzka, M.A. High-value products from microalgae—Their development and commercialisation. J. Appl. Phycol. 2013, 25, 743–756.
- Balskus, E.P.; Walsh, C.T. The genetic and molecular basis for sunscreen biosynthesis in cyanobacteria. Science 2010, 329, 1653–1656.
- Rastogi, R.P.; Sonani, R.R.; Madamwar, D. Cyanobacterial sunscreen scytonemin: Role in photoprotection and biomedical research. Appl. Biochem. Biotechnol. 2015, 176, 1551–1563.
- Rastogi, R.P.; Madamwar, D. Cyanobacteria synthesize their own UV-sunscreens for photoprotection. Bioenergetics 2016, 5, 1000138.
- Derikvand, P.; Llewellyn, C.A.; Purton, S. Cyanobacterial metabolites as a source of sunscreens and moisturizers: A comparison with current synthetic compounds. Eur. J. Phycol. 2017, 52, 43–56.
- Valiente, E.F.; Ucha, A.; Quesada, A.; Leganés, F.; Carreres, R. Contribution of N2 fixing cyanobacteria to rice production: Availability of nitrogen from 15N-labelled cyanobacteria and ammonium sulphate to rice. Plant Soil 2000, 1, 107–112.
- Prasanna, R.; Sharma, E.; Sharma, P.; Kumar, A.; Kumar, R.; Gupta, V.; Pal, R.K.; Singh Shivay, Y.; Nain, L. Soil fertility and establishment potential of inoculated cyanobacteria in rice crop grown under non-flooded conditions. Paddy Water Environ. 2013, 11, 175–183.
- Singh, J.S.; Kumar, A.; Rai, A.N.; Singh, D.P. Cyanobacteria: A precious bio-resource in agriculture, ecosystem, and environmental sustainability. Front. Microbiol. 2016, 7, 529.
- Gonçalves, A.L. The use of microalgae and cyanobacteria in the improvement of agricultural practices: A review on their biofertilising, biostimulating and biopesticide roles. Appl. Sci. 2021, 11, 871.
- Henrikson, R. A nutrient rich supper food for super health. In Spirulina World Food; Henrikson, R., Ed.; Ronore Enterprises, Inc.: Hawaii, HI, USA, 2010; pp. 25–42.
- García, J.L.; de Vicente, M.; Galán, B. Microalgae, old sustainable food and fashion nutraceuticals. Microb. Biotechnol. 2017, 10, 1017–1024.
This entry is offline, you can click here to edit this entry!
