Blood cancers are a type of liquid tumor which means cancer is present in the body fluid. Multiple myeloma, leukemia, and lymphoma are the three common types of blood cancers. Chemotherapy is the major therapy of blood cancers by systemic administration of anticancer agents into the blood. However, a high incidence of relapse often happens, due to the low efficiency of the anticancer agents that accumulate in the tumor site, and therefore lead to a low survival rate of patients. This indicates an urgent need for a targeted drug delivery system to improve the safety and efficacy of therapeutics for blood cancers.
1. Introduction
Cancers are one of the leading causes of death in the world [
1]. Unlike solid tumors such as those in organs, blood cancers (including multiple myeloma, leukemia, and lymphoma) form in the bone marrow or in the lymphatic system [
2,
3]. Although, many chemotherapeutic drugs are clinically available for the treatment of blood cancers, there are no curative treatment approaches in clinical practice for these types of cancers due to the inevitable aggravation of blood cancers and bone metastasis [
5]. Furthermore, it is difficult to achieve a sufficient therapeutic dose of anticancer agents at tumor sites inside bone marrow or the lymphatic system to suppress tumor growth via systematic administration [
6]. To maintain therapeutic levels in bone marrow or the lymphatic system, chemotherapeutics require high dosage and/or more frequent administration which can result in increased side effects [
7]. In addition, the bone marrow microenvironment contains a huge number of hematopoietic stem/progenitor cells which are resistance to chemotherapy and mediate disease refractory/relapse [
5]. Therefore, a targeted drug delivery system for blood cancers is a significant challenge for chemotherapy.
2. Targeting Delivery Strategy
2.1. Targeting Bone Marrow and Its Microenvironment
The bone marrow microenvironment plays a critical role in the maintenance of cell renewal and differentiation, especially for cancer cells. The bone marrow contains numerous blood vessels and capillaries. It is considered to be one of the most complex systems comprising various cell types including endothelia cells, stromal cells, osteocytes, fibroblasts, mesenchymal stem cells, macrophages, osteoclasts, and osteoblasts. Moreover, the non-cellular component including the extracellular matrix, oxygen tension, cytokines, and mechanical forces are also essential for cancer cell proliferation and are related to resistance. Targeting the bone marrow microenvironment can be improved by drug delivery systems and can be achieved passively or actively.
2.1.1. Passive Targeting Strategy
Potentially beneficial properties of nanotherapeutics include improved bioavailability, reduced toxicity, greater dose response, and enhanced solubility as compared with conventional medicines [8]. Passive targeting depends on accumulation of the drug delivery system at a specific end organ or tumor site, through leaky vasculatures which mainly require a delivery system for its own characteristics including the size, shape, surface zeta-potential, and other properties. In the bone marrow, the drug accumulation amounts in the bone are related to the reticulo-endothelial cells in vessels [9]. The ideal size of nanoparticles for blood cancers should be between 50 nm and 100 nm.
2.1.2. Targeting Bone Surface-Mediated Bone Marrow
2.1.2. Targeting Bone Surface-Mediated Bone Marrow
Bone is rich in hydroxyapatite which has a high affinity with glutamic acid or aspartic acid [
15]. It has been reported that several oligopeptides have demonstrated their particular interactions with bone tissues. Eight repetitive aspartic acids, also known as Asp8, is one of the successful examples which has been reported to bind to bone-resorption surfaces [
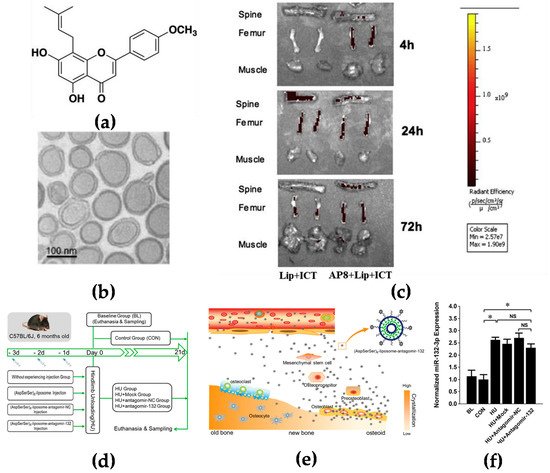
16]. Asp8 has been demonstrated to mainly bind to the highly crystallized hydroxyapatite of bone-resorption surfaces. It was found that Asp8-icaritin-liposome enhanced bone formation in ovariectomized mice as compared with an icaritin-liposome control lacking the Asp8 moiety (
Figure 1a–c) [
17]. (AspSerSer)
6 is another type of oligopeptide used for the bone-surface delivery that mainly binds to calcium phosphate which is mainly distributed in the mantle dentin in the bone [
18]. Hu et al. used the (AspSerSer)
6-cationic liposome system to deliver miRNA-132-3p in bone, resulting in prevention and treatment of osteoporosis (
Figure 1d–f) [
19].
Figure 1. Targeting the bone surface-mediated bone marrow: (
a) Characterization of the bone-targeting delivery system in vitro and in vivo. Chemical structure of icaritin (ICT); (
b) the morphology of Asp8-liposome-icaritin taken by cryo-transmission electron microscope (Cryo-TEM) (scale bar = 100 nm, magnification = 10
5×); (
c) localization of fluorescent-labeled liposome delivery system with or without Asp8 targeting peptide in mice by optical imaging IVIS analysis, 72 h after administration; (
d) a schematic diagram to illustrate the experimental design for targeted delivery of antagomir-132 to specifically decrease miRNA-132-3p levels in bone; (
e) a schematic diagram to illustrate how antagomir-132 is selectively delivered to bone formation region by (AspSerSer)
6; (
f) analysis of miRNA-132-3p expression in the femur bone tissues of mice after hindlimb unloading for 21 days. BL—baseline group, mice were euthanatized and sampled at the beginning of experiment; CON—control group mice were raised in normal conditions during the experiment; HU—hindlimb unloading group, mice were submitted to a hindlimb unloading experiment; HU + Mock—hindlimb unloading plus (AspSerSer)
6-liposome injection group, mice were injected with the (AspSerSer)
6-liposome before HU; HU + antagomir-NC—hindlimb unloading plus (AspSerSer)
6-liposome-antagomir-NC injection group, mice were injected with the (AspSerSer)
6-liposome-antagomir-NC before HU; HU + antagomir-132—hindlimb unloading plus (AspSerSer)
6-liposome-antagomir-132 injection group, mice were injected with the (AspSerSer)
6-liposome-antagomir-132 before HU. Values are shown as mean ± SD,
n = 6. *
p < 0.05. NS, not significant. (
a–
c) Adapted with permission from [
17] and (
d–
f) adapted with permission from [
19].
2.1.3. Active Targeting
Active targeting increases specific delivery to tumor tissues. Many blood cancer cells express specific surface biomarkers which can be specifically targeted by coupling peptides/antibodies/ligands to the surface of nanomedicines for drug delivery [
23,
24]. Targeting nanomedicines modified with peptides is a common strategy investigated extensively in drug delivery research. Liposomes modified with RGD peptide (Arg-Gly-Asp) have been widely used to target angiogenic endothelial cells in tumors [
25]. It has also been reported that liposomes conjugated with a cyclic pentamer peptide (VLA-4, very late antigen-4) can be used to target multiple myeloma (
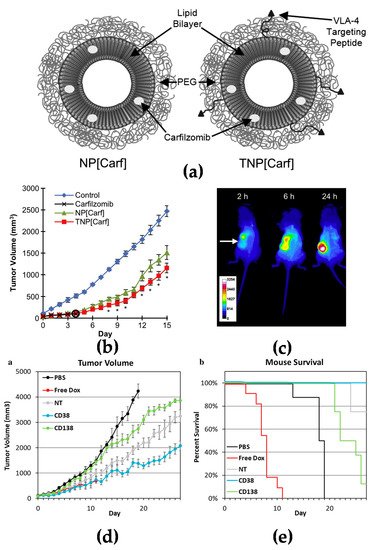
Figure 2a–c) [
26]. Targeting via antibodies is another approach for an active targeting strategy [
27]. It is known that CD38 and CD138 are widely expressed on multiple myeloma cells. Liposomes modified with anti-CD38 or anti-CD138 monoclonal antibody could be a new approach for a targeted delivery system with both targeting myeloma cells and also delivery anticancer agents to cancer cells (
Figure 2d,e) [
28]. Similar to CD38 and CD138, CD19 is one of the markers expressed in most of the lymphoma diseases [
29]; an anti-CD19 targeted liposome encapsuled rapamycin showed promising lymphoma cell-specific treatment inducing autophagic cell death [
30,
31].
Figure 2. Active targeting strategy by peptide or antibody mediated nanomedicines: (
a) Illustration of PEGylated non-targeted liposomal carfilzomib nanoparticles (NP[Carf], left) and VLA-4 targeted liposomal carfilzomib nanoparticles (TNP[Carf], right); (
b) liposomal carfilzomib nanoparticles preferentially accumulate in the tumor, inhibit tumor growth, and reduce systemic toxicities in vivo. Tumor bearing SCID mice were injected intravenously on Days 1, 2, 8, and 9 with NP[Carf], TNP[Carf], free carfilzomib, and PBS at a dose of 5 mg/kg carfilzomib equivalence. Tumor growth inhibition was measured via calipers; (
c) in vivo images of near infrared dye loaded targeted nanoparticles in tumor bearing mice. Images were taken for all mice at t = 2, 6, and 24 h using non-invasive methods. The representative images show the accumulation of the nanoparticles in the tumor (white arrow) over time; (
d,
e) in vivo efficacy of CD38pep- and CD138pep-targeted nanoparticles loaded with prodrug doxorubicin. Nanoparticles targeted with CD38pep or CD138pep were prepared loaded with a doxorubicin prodrug and their in vivo efficacy was tested against that of free doxorubicin in a subcutaneous xenograft mouse model. Mice were injected with H929 cells and tumors were allowed to grow to a predetermined size before i.v. injection of nanoparticle formulations began on Day 1. Mice were injected with 3 mg/kg of doxorubicin or nanoparticle prodrug equivalent on Days 1, 3, 5, 7, and 9. Tumor volume (
d) and survival (
e) were tracked with mice being killed when tumor volume grew too large or mouse weight was too low.
n = 6 for all groups and data represent means (± s.e.m.). (
a–
c) Adapted with permission from [
26] and (
d–
e) adapted with permission from [
28]).
2.2. Targeting Spleen and Lymphoid Nodes
Spleen and lymph nodes provide a distinct microenvironment for tumor cells in blood cancers. The spleen is considered to be involved in many blood cancers, especially in lymphomas. It has been reported that the spleen also plays a key role in tumor immunity by recruiting monocytes and macrophages to the tumor tissues [
32]. In vivo experiments have shown that siRNA encapsuled nanoparticles can reduce tumor growth [
35]. Enhanced drug concentration in the spleen has also provided therapeutic benefits in spleen resident infections and hematological disorders including malaria, hairy cell leukemia, idiopathic thrombocytopenic purpura, and autoimmune hemolytic anemia [
36].
Lymph nodes initiate most immune responses which can prevent malignant transformation [
37]. Antitumor immune responses are still active in some malignancies, impacting progression and outcome. In addition, the cytokines in lymphoid nodes also provide a proinflammatory microenvironment which can also support proliferation of malignant cells [
38].
2.3. Targeting Vascular System
Neovascularization is always associated with poor prognosis in most blood cancers including acute myeloid leukemia, multiple myeloma, acute lymphatic leukemia, chronic lymphatic leukemia, and Burkett’s lymphoma [
39]. Endothelial surface receptors are highly expressed on the inner lining of blood vessels. Shamay et al. reported that vascular endothelial growth factor receptor 1 (VEGFR1)-targeted polymer drug conjugates showed efficient antitumor effect by targeting tumor vasculature [
40]. Another strategy is to utilize tumor-homing immunocytokines such as interleukin-2 (IL-2) [
41]. The antibody-based delivery of IL-2 to extracellular targets expressed in the easily accessible tumor-associated vasculature showed therapeutic potential for acute myeloid leukemia and other solid tumors [
42].
3. Nanomedicines for Blood Cancers
3.1. Multiple Myeloma
Multiple myeloma (MM) is a B cell malignancy disease which is characterized by the accumulation of malignant plasma cells in the bone marrow. Although the new treatment and transplant has been utilized in recent decades and has prolonged the overall survival for patients, multiple myeloma is still not curable since it is difficult to remove the tumor cells from the bone marrow. Swami et al. reported that PEG-PLGA encapsuled bortezomib nanoparticles inhibited myeloma growth in a mouse model [5]. Ashley et al. reported that carfilzomib-loaded liposomal nanoparticles targeted myeloma cells [26]. A doxorubicin liposome combined with bortezomib for the treatment of relapsed or refractory multiple myeloma has already been approved by FDA for clinical use [45]. The outcome was based on a phase III clinical trial which showed that liposomal doxorubicin was superior to bortezomib monotherapy [46].
3.2. Acute Myeloid Leukemia
Acute myeloid leukemia (AML) is another common type of hematological malignancy which is characterized by high proliferation of abnormal myeloblasts in the bone marrow [
50,
51]. Chemotherapy is still the primary choice for AML treatment. However, the overall survival of single chemotherapy for AML patients is still very low [
52]. A combination of two or more anticancer reagents is often used for AML therapy to increase the treatment outcome, but various adverse effects can happen during the treatments [
53]. Recently, there are some drug delivery systems that have been investigated to increase the anti-AML effect. Roboz et al. reported on a lipid-drug conjugate encapsuled cytarabine that has been put into a phase III clinical trial [
54]. Alakhova et al. reported on a pluronic-based micelle which could increase the anti-AML efficacy of doxorubicin and was also in a phase III clinical trial [
55]. Tardi et al. reported on a cytarabine liposome which could increase accumulation in leukemia cells inside the bone marrow and enhance efficacy in AML xenograft model [
56].
3.3. B Cell Lymphomas
Lymphoma is a type of cancer which often happens in lymph nodes. The majority arise from B cells, and therefore, are called B cell lymphomas which include both Hodgkin’s lymphomas and most non-Hodgkin lymphomas [
60]. Chemotherapy and stem cell transplantation are two main treatments for B cell lymphomas; however, relapse is often inevitable [
61]. Antibody conjugates provided a new way for targeting therapy for B cell lymphomas. Brentuximab vedotin (Adcetris
®, Seattle Genetics, Bothell, WA, USA) and ibritumomab tiuxetan (Zevalin
®, IDEC, Cambridge, MA /Spectrum, Irvine, CA, USA) are two commercially available antibody-drug conjugates for Hodgkin lymphoma and non-Hodgkin lymphoma which have already been approved by the FDA [
62,
63]. Furthermore, new technology provides the possibility to selectively deliver anticancer agents to malignant cells without damaging healthy cells or systemic toxicity, allowing them to reach the lymph nodes. Nevala et al. reported on a nano-antibody targeted chemotherapy delivery system that used a slight modification of existing cancer drugs with significantly improved treatment efficacy in CD20+ B-cell lymphoma [
64].
This entry is adapted from the peer-reviewed paper 10.3390/molecules27041310