The health benefits of fibre consumption are sound, but a more compressive understanding of the individual effects of different fibres is still needed. Arabinoxylan is a complex fibre that provides a wide range of health benefits strongly regulated by its chemical structure. Arabinoxylans can be found in various grains, such as wheat, barley, or corn.
- arabinoxylans
- dietary fibre
- health benefits
1. Introduction
2. Structure of Arabinoxylan
| Source of Arabinoxylan | Tissue Type | Total AXs (%) | WEAXs (%) | References | Main AX Structure * | References |
|---|---|---|---|---|---|---|
| Wheat | Endosperm | 1.52–1.75 | 0.42–0.68 | [14] | Side chains linked by α-(1→2) and/or α-(1→3) bonds along the xylan backbone. Xyloses are most commonly mono-substituted. Side chains formed mainly by single arabinose units but can contain other short sugar sidechains. |
[12][15][16][17] |
| Bran | 11.0–16.4 | 0.54–0.95 | [14] | |||
| Barley | Endosperm | 1.2–1.3 | 0.42–0.47 | [18] | Similar structure to wheat AXs. Side chains of xylose units in the 2 and/or 3 carbon of the xyloses, which form the backbones of these AXs. Consists of more arabinose side chains than wheat AXs. | [19][20][21][22] |
| Bran | 10.26 | - | [22] | |||
| Corn | Cob | 26.24 | - | [23] | Highly branched structures with a xylose backbone. Side chains of arabinose residues on primary and secondary hydroxyl groups. Glucuronic acid, galactose, and xylose residues can also be present. | [24][25][26] |
| Bran | 26.0 | 0.71 | [27] | |||
| Rice | Endosperm | 1.83 | 0.05 | [28] | Characteristic sugar linkages and non-reducing end xylose and galactose. (1→2)-, (1→3)- or (1→5)-linked arabinose residues also present. | [29][30] |
| Bran | 6.82 | 011 | [28] | |||
| Rye | Endosperm | 3.56–4.25 | [31] | Main chain of 4-linked β-D-xylopyranosyl residues. A terminal α-L-arabinofuranosyl residue substitutes (on average) every second unit at position 3 and a small portion of the xylose units at position 2 and 3. | [32][33][34] | |
| Bran | 12.6 | 2.1 | [31] | |||
| Oat | Endosperm | 1.2 | 0.2 | [35] | (1–4)-linked β-D-xylopyranosyl residues making up the main chain, with terminal L-arabinofuranosyl residues substituting at O-3, but also at both O-2 and O-3. | [35][36] |
| Bran | 5.2 | 0.7 | [35] |
2.1. Structure of Wheat Arabinoxylan
2.2. Structure of Barley Arabinoxylan
2.3. Structure of Corn Arabinoxylan
3. Extraction and Production of AXs as a Food Ingredient
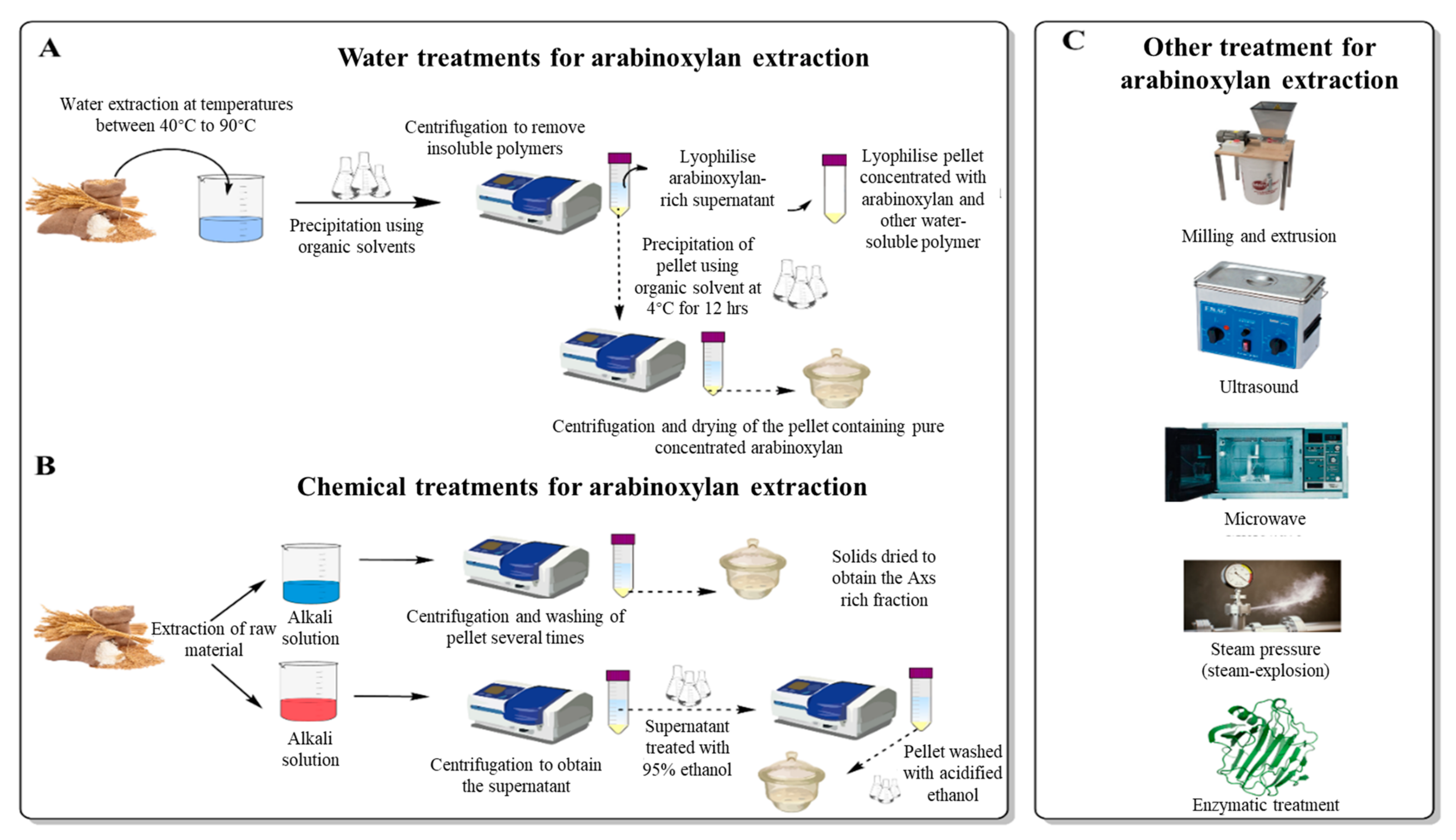
3.1. Water Extraction of Arabinoxylans
3.2. Mechanical Extraction of Arabinoxylans
3.3. Chemical Extraction of Arabinoxylans
| Source | Extraction | Solvent/Enzyme | AXs Yield * | A/X Ratio | Reference |
|---|---|---|---|---|---|
| De-starched wheat bran | Alkali | 0.44 M NaOH | 20.80 | 0.94 | [83] |
| Corn fibre | Alkali | 0.25–50 M NaOH | 26.80 ** | n.d. | [84] |
| De-starched plan materials | Alkali | NaOH (pH 11.5) | 14.30–59.9 *** | n.d. | [57] |
| Chinese, black-grained wheat bran residue (after removal of water-extractable polysaccharides) | Alkali | Saturated Ba(OH)2, 1% NaBH4 | ~5.8 | 0.6 | [85] |
| Wheat bran | Alkali | Saturated Ba(OH)2, 0.26 M NaBH4 | 24 | 0.7 | [86] |
| Corn husk | Alkali | 0.9% (w/v) Ca(OH)2 | n.d. | 0.75 | [87] |
| De-starched wheat | Alkali/Enzymatic + alkali | 0.16 mol/L NaOH, 0.5% H2O2//xylanase and cellulase (sodium acetate buffer) + 0.16 mol/L NaOH, 0.5% H2O2 | 19.83//5.27 and 14.95 | 1.14//0.25 and 1.52 | [13] |
| Rye bran | Alkali + enzymatic | First extraction: 0.17 M Na2CO3 or 0.17 M Ca (OH)2 or water Second extraction: xylanase |
First extraction: 2.92–3.85 Second extraction: 7.5–9.85 |
First extraction: 0.48–0.59 Second extraction: 0.23–0.28 |
[88] |
| Wheat and barley straw | Alkali and steam pretreatment + enzymatic | 1–2 wt% NaOH (steam pretreatment) + β-glucosidase and xylanase | 18–35 (Wheat) 17–47 (Barley) |
n.d. | [89] |
| Wheat bran | Ultrasound + Enzymatic | Xylanase (sodium acetate buffer) | 4.25–12.88 | n.d. | [66] |
| Wheat bran | Enzymatic | Xylanase | 23.1 | 0.44 | [90] |
| Corn fibre | Enzymatic | Xylanase and cellulase (sodium acetate buffer) | 30–45 | n.d. | [90] |
* AX extracted yield by raw material dry basis (% of Dw). ** Maximum yield achieved at optimized NaOH concentration, time, and temperature (0.5 M, 2 h, 60 °C). *** Yields were dependent on the material; yield could be influenced by pretreatments of these plant materials carried out by manufacturers. n.d.: not determined.
3.4. Enzymatic Extraction of Arabinoxylans
This entry is adapted from the peer-reviewed paper 10.3390/foods11071026
References
- Gill, S.K.; Rossi, M.; Bajka, B.; Whelan, K. Dietary fibre in gastrointestinal health and disease. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 101–116.
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502.
- Cunningham, M.; Azcarate-Peril, M.A.; Barnard, A.; Benoit, V.; Grimaldi, R.; Guyonnet, D.; Holscher, H.D.; Hunter, K.; Manurung, S.; Obis, D.; et al. Shaping the Future of Probiotics and Prebiotics. Trends Microbiol. 2021, 29, 667–685.
- Paesani, C.; Degano, A.L.; Salvucci, E.; Zalosnik, M.I.; Fabi, J.P.; Sciarini, L.S.; Perez, G.T. Soluble arabinoxylans extracted from soft and hard wheat show a differential prebiotic effect in vitro and in vivo. J. Cereal Sci. 2020, 93, 102956.
- Nguyen, N.K.; Deehan, E.C.; Zhang, Z.; Jin, M.; Baskota, N.; Perez-Muñoz, M.E.; Cole, J.; Tuncil, Y.E.; Seethaler, B.; Wang, T.; et al. Gut microbiota modulation with long-chain corn bran arabinoxylan in adults with overweight and obesity is linked to an individualized temporal increase in fecal propionate. Microbiome 2020, 8, 118.
- Carvajal-Millan, E.; Vargas-Albores, F.; Fierro-Islas, J.M.; Gollas-Galván, T.; Magdaleno-Moncayo, D.; Rascon-Chu, A.; Martínez-Porchas, M.; Lago-Lestón, A. Arabinoxylans and gelled arabinoxylans used as anti-obesogenic agents could protect the stability of intestinal microbiota of rats consuming high-fat diets. Int. J. Food Sci. Nutr. 2019, 71, 74–83.
- Lin, S.; Agger, J.W.; Wilkens, C.; Meyer, A.S. Feruloylated Arabinoxylan and Oligosaccharides: Chemistry, Nutritional Functions, and Options for Enzymatic Modification. Annu. Rev. Food Sci. Technol. 2021, 12, 331–354.
- Lazaridou, A.; Chornick, T.; Biliaderis, C.G.; Izydorczyk, M.S. Sequential solvent extraction and structural characterization of polysaccharides from the endosperm cell walls of barley grown in different environments. Carbohydr. Polym. 2008, 73, 621–639.
- Mudgil, D.; Barak, S. Composition, properties and health benefits of indigestible carbohydrate polymers as dietary fiber: A review. Int. J. Biol. Macromol. 2013, 61, 1–6.
- Ebringerová, A.; Heinze, T. Xylan and Xylan Derivatives—Biopolymers with Valuable Properties, 1. Naturally Occurring Xylans Structures, Isolation Procedures and Properties. Macromol. Rapid Commun. 2000, 21, 542–556.
- He, H.-J.; Qiao, J.; Liu, Y.; Guo, Q.; Ou, X.; Wang, X. Isolation, Structural, Functional, and Bioactive Properties of Cereal Arabinoxylan—A Critical Review. J. Agric. Food Chem. 2021, 69, 15437–15457.
- Izydorczyk, M.S.; Biliaderis, C. Cereal arabinoxylans: Advances in structure and physicochemical properties. Carbohydr. Polym. 1995, 28, 33–48.
- Chen, H.; Chen, Z.; Fu, Y.; Liu, J.; Lin, S.; Zhang, Q.; Liu, Y.; Wu, D.; Lin, D.; Han, G.; et al. Structure, Antioxidant, and Hypoglycemic Activities of Arabinoxylans Extracted by Multiple Methods from Triticale. Antioxidants 2019, 8, 584.
- Marcotuli, I.; Hsieh, Y.S.-Y.; Lahnstein, J.; Yap, K.; Burton, R.A.; Blanco, A.; Fincher, G.B.; Gadaleta, A. Structural Variation and Content of Arabinoxylans in Endosperm and Bran of Durum Wheat (Triticum turgidum L.). J. Agric. Food Chem. 2016, 64, 2883–2892.
- Barron, C.; Bar-L’Helgouac’h, C.; Champ, M.; Saulnier, L. Arabinoxylan content and grain tissue distribution are good predictors of the dietary fibre content and their nutritional properties in wheat products. Food Chem. 2020, 328, 127111.
- Saulnier, L.; Sado, P.-E.; Branlard, G.; Charmet, G.; Guillon, F. Wheat arabinoxylans: Exploiting variation in amount and composition to develop enhanced varieties. J. Cereal Sci. 2007, 46, 261–281.
- Saulnier, L.; Guillon, F.; Chateigner-Boutin, A.-L. Cell wall deposition and metabolism in wheat grain. J. Cereal Sci. 2012, 56, 91–108.
- Comino, P.; Shelat, K.; Collins, H.; Lahnstein, J.; Gidley, M.J. Separation and Purification of Soluble Polymers and Cell Wall Fractions from Wheat, Rye and Hull less Barley Endosperm Flours for Structure-Nutrition Studies. J. Agric. Food Chem. 2013, 61, 12111–12122.
- Li, L.-Y.; Wang, Y.-X.; Zhang, T.; Zhang, J.-F.; Pan, M.; Huang, X.-J.; Yin, J.-Y.; Nie, S.-P. Structural characteristics and rheological properties of alkali-extracted arabinoxylan from dehulled barley kernel. Carbohydr. Polym. 2020, 249, 116813.
- Izydorczyk, M.S.; Jacobs, M.; Dexter, J.E. Distribution and Structural Variation of Nonstarch Polysaccharides in Milling Fractions of Hull-less Barley with Variable Amylose Content. Cereal Chem. 2003, 80, 645–653.
- Lazaridou, A.; Chornick, T.; Biliaderis, C.G.; Izydorczyk, M.S. Composition and molecular structure of polysaccharides released from barley endosperm cell walls by sequential extraction with water, malt enzymes, and alkali. J. Cereal Sci. 2008, 48, 304–318.
- Zheng, X.; Li, L.; Wang, X. Molecular Characterization of Arabinoxylans from Hull-Less Barley Milling Fractions. Molecules 2011, 16, 2743–2753.
- Kundu, P.; Kumar, S.; Ahluwalia, V.; Kansal, S.K.; Elumalai, S. Extraction of arabinoxylan from corncob through modified alkaline method to improve xylooligosaccharides synthesis. Bioresour. Technol. Rep. 2018, 3, 51–58.
- Saulnier, L.; Marot, C.; Chanliaud, E.; Thibault, J.-F. Cell wall polysaccharide interactions in maize bran. Carbohydr. Polym. 1995, 26, 279–287.
- Montgomery, R.; Smith, F. Structure of Corn Hull Hemicellulose. Part III. Identification of the Methylated Aldobiouronic Acid Obtained from Methyl Corn Hull Hemicellulose1,2. J. Am. Chem. Soc. 1957, 79, 695–697.
- Whistler, R.L.; Corbett, W.M. Oligosaccharides from Partial Acid Hydrolysis of Corn Fiber Hemicellulose1,2. J. Am. Chem. Soc. 1955, 77, 6328–6330.
- Zhang, Z.; Smith, C.; Li, W.; Ashworth, J. Characterization of Nitric Oxide Modulatory Activities of Alkaline-Extracted and Enzymatic-Modified Arabinoxylans from Corn Bran in Cultured Human Monocytes. J. Agric. Food Chem. 2016, 64, 8128–8137.
- Hashimoto, S.; Shogren, M.D.; Bolte, L.C.; Pomeranz, Y. Cereal Pentosans: Their Estimation and Significance III Pentosans in Abraded Grains and Milling Products. Cereal Chem. 1987, 64, 39–41.
- Fadel, A.; Plunkett, A.; Li, W.; Ranneh, Y.; Gyamfi, V.E.T.; Salmon, Y.; Nyaranga, R.R.; Ashworth, J. Arabinoxylans from rice bran and wheat immunomodulatory potentials: A review article. Nutr. Food Sci. 2018, 48, 97–110.
- Shibuya, N.; Iwasaki, T. Structural features of rice bran hemicellulose. Phytochemistry 1985, 24, 285–289.
- Vinkx, J.A.; Delcour, J.A. Rye (Secale cereale L.) Arabinoxylans: A Critical Review. J. Cereal Sci. 1996, 24, 1–14.
- Aspinall, G.O.; Sturgeon, R.J. 900. Cereal gums. Part II. The constitution of an araboxylan from rye flour. J. Chem. Soc. 1957, 4469–4471.
- Åman, P.; Bengtsson, S. Periodate oxidation and degradation studies on the major water-soluble arabinoxylan in rye grain. Carbohydr. Polym. 1991, 15, 405–414.
- Nilsson, M.; Saulnier, L.; Andersson, R.; Åman, P. Water unextractable polysaccharides from three milling fractions of rye grain. Carbohydr. Polym. 1996, 30, 229–237.
- Westerlund, E.; Andersson, R.; Åman, P. Isolation and chemical characterization of water-soluble mixed-linked β-glucans and arabinoxylans in oat milling fractions. Carbohydr. Polym. 1993, 20, 115–123.
- Tian, L.; Gruppen, H.; Schols, H.A. Characterization of (Glucurono)arabinoxylans from Oats Using Enzymatic Fingerprinting. J. Agric. Food Chem. 2015, 63, 10822–10830.
- Dornez, E.; Gebruers, K.; Wiame, S.; Delcour, J.A.; Courtin, C.M. Insight into the Distribution of Arabinoxylans, Endoxylanases, and Endoxylanase Inhibitors in Industrial Wheat Roller Mill Streams. J. Agric. Food Chem. 2006, 54, 8521–8529.
- Gebruers, K.; Dornez, E.; Boros, D.; Fraś, A.; Dynkowska, W.; Bedő, Z.; Rakszegi, M.; Delcour, J.A.; Courtin, C.M. Variation in the Content of Dietary Fiber and Components Thereof in Wheats in the HEALTHGRAIN Diversity Screen. J. Agric. Food Chem. 2008, 56, 9740–9749.
- Hemery, Y.; Rouau, X.; Lullien-Pellerin, V.; Barron, C.; Abecassis, J. Dry processes to develop wheat fractions and products with enhanced nutritional quality. J. Cereal Sci. 2007, 46, 327–347.
- Kaur, A.; Yadav, M.P.; Singh, B.; Bhinder, S.; Simon, S.; Singh, N. Isolation and characterization of arabinoxylans from wheat bran and study of their contribution to wheat flour dough rheology. Carbohydr. Polym. 2019, 221, 166–173.
- Ordaz-Ortiz, J.J.; Saulnier, L. Structural variability of arabinoxylans from wheat flour. Comparison of water-extractable and xylanase-extractable arabinoxylans. J. Cereal Sci. 2005, 42, 119–125.
- Zhang, Z.; Smith, C.; Li, W. Extraction and modification technology of arabinoxylans from cereal by-products: A critical review. Food Res. Int. 2014, 65, 423–436.
- Gao, X.; Ying, R.; Huang, M. Effects of lamellar organization and arabinoxylan substitution rate on the properties of films simulating wheat grain aleurone cell wall. Carbohydr. Polym. 2021, 270, 117819.
- Philippe, S.; Barron, C.; Robert, P.; Devaux, M.-F.; Saulnier, L.; Guillon, F. Characterization Using Raman Microspectroscopy of Arabinoxylans in the Walls of Different Cell Types during the Development of Wheat Endosperm. J. Agric. Food Chem. 2006, 54, 5113–5119.
- Antoine, C.; Peyron, S.; Mabille, F.; Lapierre, C.; Bouchet, B.; Abecassis, A.J.; Rouau, X. Individual Contribution of Grain Outer Layers and Their Cell Wall Structure to the Mechanical Properties of Wheat Bran. J. Agric. Food Chem. 2003, 51, 2026–2033.
- Barron, C.; Surget, A.; Rouau, X. Relative amounts of tissues in mature wheat (Triticum aestivum L.) grain and their carbohydrate and phenolic acid composition. J. Cereal Sci. 2007, 45, 88–96.
- Parker, M.L.; Ng, A.; Waldron, K.W. The phenolic acid and polysaccharide composition of cell walls of bran layers of mature wheat (Triticum aestivum L. cv. Avalon) grains. J. Sci. Food Agric. 2005, 85, 2539–2547.
- Gartaula, G.; Dhital, S.; Netzel, G.; Flanagan, B.M.; Yakubov, G.E.; Beahan, C.T.; Collins, H.M.; Burton, R.A.; Bacic, A.; Gidley, M.J. Quantitative structural organisation model for wheat endosperm cell walls: Cellulose as an important constituent. Carbohydr. Polym. 2018, 196, 199–208.
- Maes, C.; Delcour, J. Structural Characterisation of Water-extractable and Water-unextractable Arabinoxylans in Wheat Bran. J. Cereal Sci. 2002, 35, 315–326.
- Wang, J.; Bai, J.; Fan, M.; Li, T.; Li, Y.; Qian, H.; Wang, L.; Zhang, H.; Qi, X.; Rao, Z. Cereal-derived arabinoxylans: Structural features and structure–activity correlations. Trends Food Sci. Technol. 2020, 96, 157–165.
- Viëtor, R.; Angelino, S.; Voragen, A. Structural features of arabinoxylans from barley and malt cell wall material. J. Cereal Sci. 1992, 15, 213–222.
- Trogh, I.; Courtin, C.M.; Delcour, J.A. Isolation and Characterization of Water-Extractable Arabinoxylan from Hull-less Barley Flours. Cereal Chem. 2004, 81, 576–581.
- Yadav, M.P.; Moreau, R.A.; Hicks, K.B. Phenolic Acids, Lipids, and Proteins Associated with Purified Corn Fiber Arabinoxylans. J. Agric. Food Chem. 2006, 55, 943–947.
- Yadav, M.P.; Johnston, D.B.; Hotchkiss, A.; Hicks, K.B. Corn fiber gum: A potential gum arabic replacer for beverage flavor emulsification. Food Hydrocoll. 2007, 21, 1022–1030.
- Doner, L.W.; Johnston, D.B.; Singh, V. Analysis and Properties of Arabinoxylans from Discrete Corn Wet-Milling Fiber Fractions. J. Agric. Food Chem. 2001, 49, 1266–1269.
- Doner, L.W.; Hicks, K.B. Isolation of Hemicellulose from Corn Fiber by Alkaline Hydrogen Peroxide Extraction. Cereal Chem. 1997, 74, 176–181.
- Yadav, M.P.; Kale, M.S.; Hicks, K.B.; Hanah, K. Isolation, characterization and the functional properties of cellulosic arabinoxylan fiber isolated from agricultural processing by-products, agricultural residues and energy crops. Food Hydrocoll. 2017, 63, 545–551.
- Fadel, A.; Mahmoud, A.M.; Ashworth, J.J.; Li, W.; Ng, Y.L.; Plunkett, A. Health-related effects and improving extractability of cereal arabinoxylans. Int. J. Biol. Macromol. 2018, 109, 819–831.
- Izydorczyk, M.; Macri, L.; MacGregor, A. Structure and physicochemical properties of barley non-starch polysaccharides—I. Water-extractable β-glucans and arabinoxylans. Carbohydr. Polym. 1998, 35, 249–258.
- Shang, X.-L.; Liu, C.-Y.; Dong, H.-Y.; Peng, H.-H.; Zhu, Z.-Y. Extraction, purification, structural characterization, and antioxidant activity of polysaccharides from Wheat Bran. J. Mol. Struct. 2021, 1233, 130096.
- Malunga, L.N.; Izydorczyk, M.; Beta, T. Effect of water-extractable arabinoxylans from wheat aleurone and bran on lipid peroxidation and factors influencing their antioxidant capacity. Bioact. Carbohydrates Diet. Fibre 2017, 10, 20–26.
- Jacquemin, L.; Zeitoun, R.; Sablayrolles, C.; Pontalier, P.-Y.; Rigal, L. Evaluation of the technical and environmental performances of extraction and purification processes of arabinoxylans from wheat straw and bran. Process Biochem. 2012, 47, 373–380.
- Jacquemin, L.; Mogni, A.; Zeitoun, R.; Guinot, C.; Sablayrolles, C.; Saulnier, L.; Pontalier, P.-Y. Comparison of different twin-screw extraction conditions for the production of arabinoxylans. Carbohydr. Polym. 2015, 116, 86–94.
- Demuth, T.; Betschart, J.; Nyström, L. Structural modifications to water-soluble wheat bran arabinoxylan through milling and extrusion. Carbohydr. Polym. 2020, 240, 116328.
- Andersson, A.A.; Andersson, R.; Jonsäll, A.; Andersson, J.; Fredriksson, H. Effect of Different Extrusion Parameters on Dietary Fiber in Wheat Bran and Rye Bran. J. Food Sci. 2017, 82, 1344–1350.
- Wang, J.; Sun, B.; Liu, Y.; Zhang, H. Optimisation of ultrasound-assisted enzymatic extraction of arabinoxylan from wheat bran. Food Chem. 2014, 150, 482–488.
- Reis, S.F.; Coelho, E.; Coimbra, M.A.; Abu-Ghannam, N. Improved efficiency of brewer’s spent grain arabinoxylans by ultrasound-assisted extraction. Ultrason. Sonochem. 2015, 24, 155–164.
- Görgüç, A.; Bircan, C.; Yılmaz, F.M. Sesame bran as an unexploited by-product: Effect of enzyme and ultrasound-assisted extraction on the recovery of protein and antioxidant compounds. Food Chem. 2019, 283, 637–645.
- Roos, A.A.; Persson, T.; Krawczyk, H.; Zacchi, G.; Stålbrand, H. Extraction of water-soluble hemicelluloses from barley husks. Bioresour. Technol. 2009, 100, 763–769.
- Minjares-Fuentes, R.; Femenia, A.; Garau, M.; Candelas-Cadillo, M.; Simal, S.; Rosselló, C. Ultrasound-assisted extraction of hemicelluloses from grape pomace using response surface methodology. Carbohydr. Polym. 2016, 138, 180–191.
- Coelho, E.; Rocha, M.A.M.; Saraiva, J.A.; Coimbra, M.A. Microwave superheated water and dilute alkali extraction of brewers’ spent grain arabinoxylans and arabinoxylo-oligosaccharides. Carbohydr. Polym. 2014, 99, 415–422.
- Davis, E.J.; Andreani, E.S.; Karboune, S. Production of Extracts Composed of Pectic Oligo/Polysaccharides and Polyphenolic Compounds from Cranberry Pomace by Microwave-Assisted Extraction Process. Food Bioprocess Technol. 2021, 14, 634–649.
- Kong, F.; Wang, L.; Chen, H.; Zhao, X. Improving storage property of wheat bran by steam explosion. Int. J. Food Sci. Technol. 2021, 56, 287–292.
- Aktas-Akyildiz, E.; Mattila, O.; Sozer, N.; Poutanen, K.; Koksel, H.; Nordlund, E. Effect of steam explosion on enzymatic hydrolysis and baking quality of wheat bran. J. Cereal Sci. 2017, 78, 25–32.
- Sui, W.; Xie, X.; Liu, R.; Wu, T.; Zhang, M. Effect of wheat bran modification by steam explosion on structural characteristics and rheological properties of wheat flour dough. Food Hydrocoll. 2018, 84, 571–580.
- Singla, M.; Sit, N. Application of ultrasound in combination with other technologies in food processing: A review. Ultrason. Sonochem. 2021, 73, 105506.
- Sillero, L.; Prado, R.; Labidi, J. Simultaneous microwave-ultrasound assisted extraction of bioactive compounds from bark. Chem. Eng. Process. Process Intensif. 2020, 156, 108100.
- Wen, L.; Zhang, Z.; Sun, D.-W.; Sivagnanam, S.P.; Tiwari, B.K. Combination of emerging technologies for the extraction of bioactive compounds. Crit. Rev. Food Sci. Nutr. 2020, 60, 1826–1841.
- Xu, F.; Liu, C.-F.; Geng, Z.; Sun, J.; Sun, R.; Hei, B.; Lin, L.; Wu, S.; Je, J. Characterisation of degraded organosolv hemicelluloses from wheat straw. Polym. Degrad. Stab. 2006, 91, 1880–1886.
- Fincher, G.B.; Stone, B.A. Cell walls and their components in cereal grain technology. Adv. Cereal Sci. Technol. 1986, 8, 207–295.
- Cyran, M.; Courtin, C.M.; Delcour, J.A. Heterogeneity in the Fine Structure of Alkali-Extractable Arabinoxylans Isolated from Two Rye Flours with High and Low Breadmaking Quality and Their Coexistence with Other Cell Wall Components. J. Agric. Food Chem. 2004, 52, 2671–2680.
- Greenfield, H.; Southgate, D.A.T. Review of Methods of Analysis. In Food Composition Data: Production, Management, and Use; FAO: Rome, Italy, 2003.
- Aguedo, M.; Fougnies, C.; Dermience, M.; Richel, A. Extraction by three processes of arabinoxylans from wheat bran and characterization of the fractions obtained. Carbohydr. Polym. 2014, 105, 317–324.
- Ayala-Soto, F.E.; Serna-Saldívar, S.O.; Welti-Chanes, J. Effect of processing time, temperature and alkali concentration on yield extraction, structure and gelling properties of corn fiber arabinoxylans. Food Hydrocoll. 2016, 60, 21–28.
- Sun, Y.; Cui, S.W.; Gu, X.; Zhang, J. Isolation and structural characterization of water unextractable arabinoxylans from Chinese black-grained wheat bran. Carbohydr. Polym. 2011, 85, 615–621.
- Schooneveld-Bergmans, M.; Beldman, G.; Voragen, A. Structural Features of (Glucurono)Arabinoxylans Extracted from Wheat Bran by Barium Hydroxide. J. Cereal Sci. 1999, 29, 63–75.
- Ogawa, K.; Takeuchi, M.; Nakamura, N. Immunological Effects of Partially Hydrolyzed Arabinoxylan from Corn Husk in Mice. Biosci. Biotechnol. Biochem. 2005, 69, 19–25.
- Bender, D.; Nemeth, R.; Wimmer, M.; Götschhofer, S.; Biolchi, M.; Török, K.; Tömösközi, S.; D’Amico, S.; Schoenlechner, R. Optimization of Arabinoxylan Isolation from Rye Bran by Adapting Extraction Solvent and Use of Enzymes. J. Food Sci. 2017, 82, 2562–2568.
- Persson, T.; Ren, J.L.; Joelsson, E.; Jönsson, A.-S. Fractionation of wheat and barley straw to access high-molecular-mass hemicelluloses prior to ethanol production. Bioresour. Technol. 2009, 100, 3906–3913.
- Mathew, S.; Karlsson, E.N.; Adlercreutz, P. Extraction of soluble arabinoxylan from enzymatically pretreated wheat bran and production of short xylo-oligosaccharides and arabinoxylo-oligosaccharides from arabinoxylan by glycoside hydrolase family 10 and 11 endoxylanases. J. Biotechnol. 2017, 260, 53–61.
- Ma, F.; Li, X.; Yin, J.; Ma, L.; Li, D. Optimisation of double-enzymatic extraction of arabinoxylan from fresh corn fibre. J. Food Sci. Technol. 2020, 57, 4649–4659.
- Escarnot, E.; Aguedo, M.; Paquot, M. Enzymatic hydrolysis of arabinoxylans from spelt bran and hull. J. Cereal Sci. 2012, 55, 243–253.
