In recent years, the link between diet and human health has become more widespread. The positive effects of fibres on the population’s health are becoming more apparent, although there is still more to learn. A clear relationship between the different characteristics of fibres (e.g., structure, solubility, viscosity, etc.) and their health benefits is still unclear
[1]. Nevertheless, there is a consensus that fibres act as prebiotics and positively affect human health. Prebiotics are compounds that stimulate bacteria’s growth and correlate with reducing diseases caused by a shift in the microbiota
[2][3]. This effect has been proven in different studies, such as the ones conducted by Paesani et al.
[4], Nguyen et al.
[5], and Carvajal-Millan et al.
[6], among others. Many fibres have a prebiotic effect
[1]. Among these are arabinoxylans (AXs). AXs are polysaccharides present in the cell walls of different plant tissues. They are composed of a linear backbone of xylose units linked by β1-4 bonds with arabinose units linked to some of the xylose units. Moreover, the xylose units can carry methyl-glucuronic acids, and arabinose units can bond to ferulate residues
[7]. However, the structural characteristics of different AXs are complex and influenced by their source. AXs present a wide range of water solubility (soluble and insoluble AXs), which depends on factors such as the average degree of polymerisation (DPav), degree of branching, and monomeric composition
[8][9]. Their ability to link to other polymers found in plant tissues (such as hemicellulose) also influences their solubility, reducing their extractability with water
[10].
2. Structure of Arabinoxylan
As previously stated, arabinoxylan (AX) is a polysaccharide present in the cell wall of various cereals, such as wheat, corn, rye, barley, rice, and oat
[11], and it is composed of a linear backbone of xylose units with linked arabinose units. More precisely, the general structure of an AX consists of a linear β-(1→4) linked xylan backbone to which α-l-arabinofuranose units are attached as side residues via α-(1→3) and/or α-(1→2) linkages
[12]. The molecular structure of AX is also dependent on the extraction method applied. AXs can be extracted using chemical, enzymatic, or physical treatments
[13]. A wide range of AXs are found in different plants; this entry will discuss the structure of AXs present in three primary sources: wheat, barley, and corn.
Table 1 summarises the structural characteristics of AXs from these main cereal grains, including wheat, barley, corn, rice, sorghum, rye, and oat.
Table 1. Summary of the main structural characteristics of total AXs and water-extractable AXs (WEAXs) found in different cereal grains. Xylan backbone substitutions between each AX differs. However, similar chemical structures are apparent amongst them. * The minor changes in these structural characteristics result in different interactive behaviours with other macromolecules.
| Source of Arabinoxylan |
Tissue Type |
Total AXs (%) |
WEAXs (%) |
References |
Main AX Structure * |
References |
| Wheat |
Endosperm |
1.52–1.75 |
0.42–0.68 |
[14] |
Side chains linked by α-(1→2) and/or α-(1→3) bonds along the xylan backbone.
Xyloses are most commonly mono-substituted.
Side chains formed mainly by single arabinose units but can contain other short sugar sidechains. |
[12][15][16][17] |
| Bran |
11.0–16.4 |
0.54–0.95 |
[14] |
| Barley |
Endosperm |
1.2–1.3 |
0.42–0.47 |
[18] |
Similar structure to wheat AXs. Side chains of xylose units in the 2 and/or 3 carbon of the xyloses, which form the backbones of these AXs. Consists of more arabinose side chains than wheat AXs. |
[19][20][21][22] |
| Bran |
10.26 |
- |
[22] |
| Corn |
Cob |
26.24 |
- |
[23] |
Highly branched structures with a xylose backbone. Side chains of arabinose residues on primary and secondary hydroxyl groups. Glucuronic acid, galactose, and xylose residues can also be present. |
[24][25][26] |
| Bran |
26.0 |
0.71 |
[27] |
| Rice |
Endosperm |
1.83 |
0.05 |
[28] |
Characteristic sugar linkages and non-reducing end xylose and galactose. (1→2)-, (1→3)- or (1→5)-linked arabinose residues also present. |
[29][30] |
| Bran |
6.82 |
011 |
[28] |
| Rye |
Endosperm |
3.56–4.25 |
|
[31] |
Main chain of 4-linked β-D-xylopyranosyl residues. A terminal α-L-arabinofuranosyl residue substitutes (on average) every second unit at position 3 and a small portion of the xylose units at position 2 and 3. |
[32][33][34] |
| Bran |
12.6 |
2.1 |
[31] |
| Oat |
Endosperm |
1.2 |
0.2 |
[35] |
(1–4)-linked β-D-xylopyranosyl residues making up the main chain, with terminal L-arabinofuranosyl residues substituting at O-3, but also at both O-2 and O-3. |
[35][36] |
| Bran |
5.2 |
0.7 |
[35] |
2.1. Structure of Wheat Arabinoxylan
Wheat AXs are present in endosperm (3–5% of total endosperm), aleurone, and bran cell walls (approximately 60–70% of the entire cell wall)
[12][15]. In the specific case of wheat bran, AXs represent between 10.9 and 26% of all the bran fractions
[37][38][39][40].
In wheat AXs, side chains are linked by α-(1→2) and/or α-(1→3) bonds along the xylan backbone. The xyloses can be di-substituted, mono-substituted (the most common substitution), or not substituted at all
[16][17]. These side chains are mainly formed by single arabinose units (α-l-arabinofuranose), but side chains linked to xyloses of α-d-glucuronic acid (and its methyl ether, 4-O-methyl-glucuronic acid) also occur
[16]. The structure of wheat AXs presents a wide variability, as reported by several authors
[40][41][42]. These differences are influenced by the wheat variety and the wheat grains’ maturation stage. It has been reported that the arabinose/xylose ratio decreases upon maturation
[43][44], having a positive influence on wheat AX’s water solubility
[45] According to Barron et al.
[39], AXs of the endosperm present a higher water solubility than AXs from bran, as well as a lower arabinose/xylose ratio (A/X) (~0.6) than that of AXs derived from bran (~1). Other studies confirm these findings
[46][47][48]. However, Kaur et al.
[40] reported A/X ratios to be considerably lower than 1 for wheat brans of four different wheat varieties (between 0.33 and 0.62). These authors also reported different A/X ratios for bran fractions rich in AXs. They found A/X ratios between 0.09 and 1.37 (for water-extractable fractions), 0.33 and 1.82 (for alkali-extractable fractions), and 0.38 and 0.7 (for cellulosic arabinoxylans). This variability is a good indication of the complexity and variability of wheat AX’s structure. However, it seems clear that the A/X ratio is lower for AXs located in the endosperm than for those located in other parts of wheat grains. The A/X ratio plays an important role in modulating the hydration and swelling capacity of AX
[49]. Maes and Delcour
[50] observed that wheat AX extracted from wheat bran had an A/X ratio of 0.45, but the gradual precipitation of AX with ethanol changed the ratio significantly from 0.31 to 0.85, depending on the percentage of ethanol used, demonstrating the influence that the type of extraction method can have on the A/X ratio. In addition to the xyloses, arabinoses and α-d-glucuronic acid units that form part of the AX’s other short sugar side chains can also be present in wheat AX’s structure. These side chains are constituted by xylopyranosyl and galactopyranosyl residues associated with arabinofuranosyl residues
[16]. Additionally, arabinose units/chains can also carry acetic acid and hydroxycinnamic acids (ferulic and p-coumaric esters)
[16][45].
2.2. Structure of Barley Arabinoxylan
The basic structure of barley AXs is the same as that of wheat AXs (polysaccharides mainly composed of xylose and arabinose). However, there are some notable differences. For example, barley AXs present side chains of xylose units in the 2 and/or 3 C of the xyloses, forming the backbones of AXs
[19][20]. On average, barley AXs have a higher A/X ratio than wheat AXs
[51], since their arabinose side chains are more numerous. The molecular weight (Mw) of barley AXs is also distributed in a wide range for kDa
[19][22][52], having a higher Mw for water-soluble AXs
[22]. Barley AXs are distributed along all the grain, representing around ~10–14% and ~1.2–1.3% of the bran fraction and endosperm, respectively
[20][22], and around 25–40% of barley cell walls
[8]. Evidence supports a positive relationship between higher A/X ratios (implying more branching) and improved water solubility. Izydorczyk et al.
[20] reported both AX’s higher solubility and higher A/X ratios (from the water-soluble AXs) from bran fractions (~0.8–1) than from an endosperm fraction (~0.65–75)
[20]. However, when comparing the A/X ratio of water-soluble and -insoluble AXs, these authors observed that insoluble AXs from the endosperm had a higher A/X ratio than that of soluble AXs. In disagreement with these results, Lazaridou et al.
[8] reported a higher A/X ratio for water-soluble AXs than for non-water-soluble AXs originating from the endosperm. These differences between studies could be related to the barley variety investigated, the DPav of the AXs, the germination state, or the nature of the other polymers in the grain, among other causes. In such regards, Izydorczyk et al.
[20] found a relationship between starch structure and AX’s solubility, reporting a positive relationship between the water solubility of these carbohydrates and the amylose content of the starch of barley grains. In addition, these same authors reported AXs with higher ferulic acid content in high amylopectic grains.
2.3. Structure of Corn Arabinoxylan
Corn is also a good source of AXs, although it is much less studied than AXs from wheat or barley
[24][53][54]. Around 51% of corn bran has been identified as AXs, or 67% if residual starch is not considered
[24]. However, other authors have reported lower yields of AXs from corn bran (around 35–40%)
[55][56]. These AXs have a highly branched structure with a xylose backbone and arabinose residues as side chains on primary and secondary hydroxyl group structures, with an A/X ratio of around 0.6
[24]. Glucuronic acid (linked to the o-2 position of the xylose forming the backbone), galactose (linked to the arabinose branches), and some xylose residues also form part of corn AX’s structures
[24][25][26]. In addition to this, p-coumaric acid, ferulic acid, and acetic acid have also been found to be esterified to the monomers forming the corn AXs
[24].
3. Extraction and Production of AXs as a Food Ingredient
Extraction of AXs from cereals can be performed using various techniques from different parts of the grains. The most common source from which AXs are extracted is cereal brans, where the concentration of AXs is greatest (between 10 and 25% of the total bran)
[20][22][37][39][40]. Extraction of AXs can be performed by water treatments, mechanical treatments, chemical treatments, enzymatic treatments, or by combining these techniques
[12][13][42][57][58].
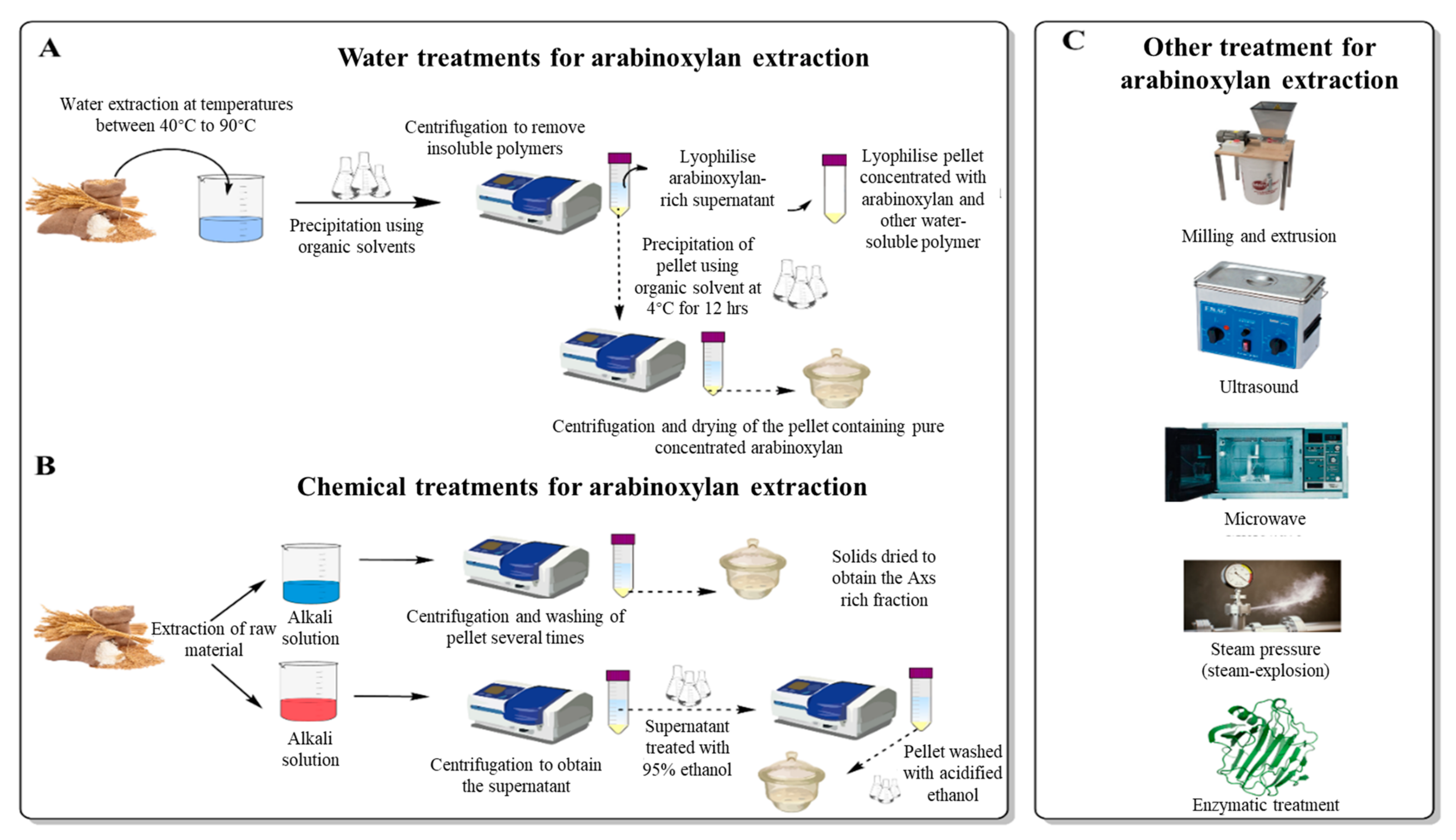
Figure 1 illustrates the different treatments that can be performed for AX’s extraction, including water and chemical treatments and other mechanical approaches.
Figure 1. Schematic illustration of a water treatment approach (A) to extract AXs from cereal grains. (B) demonstrates a different approach using acidic or basic chemical solutions to extract AXs. Other treatments (C), including mechanical (milling and extrusion, steam-pressure, ultra-sound, microwave) and enzymatic treatments, are also included.
3.1. Water Extraction of Arabinoxylans
Water extraction of AXs is the easiest and least aggressive extraction method capable of preserving AX’s native structure. As previously discussed, the water solubility of AX is dependent on several factors, such as the type of grain, the degree of germination, and the nature of the polymers forming the grain
[8][9]. These factors will undoubtedly impact the yield of AXs when extracting with water.
The extraction procedure involves solubilising the AXs by placing the milled grains (or grain fractions) in water at temperatures that can range from 45 to 90 °C for a fixed time (usually longer when using lower temperatures)
[8][43][59][60]. This solution will then be precipitated using an organic solvent. To inactivate the grains’ endogenous enzymes, samples can also be pre-treated with an aqueous ethanol solution (80% v/v)
[8]. After extraction, insoluble polymers are removed by centrifugation. The supernatant rich in AXs can be directly lyophilized to retain a pellet rich in AXs
[8] and other water-soluble polymers. To overcome this, an alternative step following the first centrifugation can be performed. The AXs in the supernatant can be precipitated with 95% ethanol or another organic solvent at around 4 °C for a fixed time (typically 12 h), followed by centrifugation and drying steps
[4][60]. Before measurement, the lyophilized sample can be treated to remove denatured proteins by filtration with celite or an equivalent compound (e.g., Fuller’s earth), and by adsorption on Vega clay (or equivalent) for the residual non-denatured proteins
[50][59][61]. Depending on the raw material used for the extraction, removing other polymers such as starch and other carbohydrates may be required. Removal is typically achieved using specific enzymes that target these polymers. Free sugar is then removed using dialysis while the enzymes are heat-inactivated
[59]. These proteins and non-AX carbohydrate removal steps can also be achieved before the lyophilisation of the pellet rich in AXs
[59]. The main limiting factor of these extraction methods is that the crosslinks between potentially soluble AXs and other polymers of the cell wall matrix are not broken, limiting the extraction yield
[12]. Thus, it might be more appealing to couple water extraction of AXs with mechanical treatments to increase solubility. The following paragraph reviews the most critical mechanical treatments to improve AX’s extractability.
3.2. Mechanical Extraction of Arabinoxylans
Mechanical extraction helps to improve the extraction yield by making AXs more accessible. In addition, other mechanical treatments are available, such as milling and extrusion
[62][63][64][65], ultrasound
[66][67][68], microwave
[69][70][71], or steam-pressure
[64]. Milling and extrusion of cereal flours/bran before an AX extraction can increase the yield of water-extractable AXs. However, such mechanical treatments can also affect the structure of AXs, reducing the substitution degree of AX significantly
[64]. The application of ultrasound technology is another successful approach for AX extraction. This technology can substantially reduce the time required (from hours to minutes) to achieve a targeted yield
[66][68]. It is essential to control the power used, as relatively high ultrasonic power can negatively affect the extraction yield
[68]. Microwave technology is a unique approach that can improve AX extractions
[69][70][71]. Compared to conventional heating methods, microwave-assisted extractions can help reduce extraction times, increase efficiency, reduce solvent consumption (if applied), and lower energy requirements
[72]. Davis et al.
[72] recently reported that the microwave extraction of polysaccharides could affect the Mw distribution of the extracted carbohydrates and the relative abundance of different polysaccharides in the final extract. Unfortunately, there is not enough research on the effect of microwave extraction of AX, as available literature often focuses on polysaccharides as a whole. Hence, more research is required to better understand the impact of microwave extraction on AX. Steam-pressure application to stabilize against spoiling flours or bran can also positively affect AX’s extractability. For example, according to Kong et al.
[73], steaming bran improved soluble fibre extractability as the soluble dietary fibre percentage increased from 4.57 to 9.10%; this was in agreement with Aktas-Akyyildiz et al.
[74], who reported an increased water extractability of AX (from 0.75 to 2.06%) after steam-pressure treatment. Similarly, Sui et al.
[75] reported that steam pressure could transform some insoluble dietary fibre into soluble fibre, thus improving water extractability. Another approach that is becoming more common in the literature is the combination of different mechanical treatments
[76][77][78]. However, there is still a lot to be understood about the combination of different mechanical treatments for better AX extractability, as research related to this is still limited.
3.3. Chemical Extraction of Arabinoxylans
Chemical extraction of arabinoxylans can be performed using alkali or acidic solution and has been well-reviewed by Zhang et al.
[42]. The chemical extraction procedure of AX consists of submerging the raw material in the chemical solution and extracting for a set period using specific conditions. After extraction, the solid residue needs to be separated, which is achieved by centrifugation
[57][79]. When the extraction is performed using an alkali solvent (sodium hydroxide (NaOH)), the pellet is washed several times to remove undesirable compounds. The solids are then dried to obtain the AX’s rich fraction
[57]. When using an acid solvent, the pellet is discarded, and the supernatant is treated with three times the volume of 95% ethanol to achieve hemicellulose precipitation. The pellet is then separated by centrifugation and washed with acidified 70% ethanol before drying to obtain an AX’s rich fraction
[79]. Depending on the chemical used for the extraction, these steps might vary. Alkali solvents can disrupt covalent and hydrogen bonds and loosen up cell wall matrixes, which results in a release of polysaccharides present in the cell wall that cannot be extracted with just water
[80][81]. Alkaline solvents can also change the charge of uronic acid residues to their negative form, favouring repulsion forces that improve AX extractability
[82].
Table 2 summarises some of the available studies that use different alkali solvents to extract AXs. Acid solvents for AX extraction are not as common as alkali solvents because they can have a hydrolysing effect on the AXs of interest. If the chemical hydrolysis is extensive, some AXs may be degraded into very low-Mw AXs that dissolve in the organic solvent, leading to a decreased yield
[42][81].
Table 2. Various arabinoxylans extraction procedures and outcomes.
| Source |
Extraction |
Solvent/Enzyme |
AXs Yield * |
A/X Ratio |
Reference |
| De-starched wheat bran |
Alkali |
0.44 M NaOH |
20.80 |
0.94 |
[83] |
| Corn fibre |
Alkali |
0.25–50 M NaOH |
26.80 ** |
n.d. |
[84] |
| De-starched plan materials |
Alkali |
NaOH (pH 11.5) |
14.30–59.9 *** |
n.d. |
[57] |
| Chinese, black-grained wheat bran residue (after removal of water-extractable polysaccharides) |
Alkali |
Saturated Ba(OH)2, 1% NaBH4 |
~5.8 |
0.6 |
[85] |
| Wheat bran |
Alkali |
Saturated Ba(OH)2, 0.26 M NaBH4 |
24 |
0.7 |
[86] |
| Corn husk |
Alkali |
0.9% (w/v) Ca(OH)2 |
n.d. |
0.75 |
[87] |
| De-starched wheat |
Alkali/Enzymatic + alkali |
0.16 mol/L NaOH, 0.5% H2O2//xylanase and cellulase (sodium acetate buffer) + 0.16 mol/L NaOH, 0.5% H2O2 |
19.83//5.27 and 14.95 |
1.14//0.25 and 1.52 |
[13] |
| Rye bran |
Alkali + enzymatic |
First extraction: 0.17 M Na2CO3 or 0.17 M Ca (OH)2 or water
Second extraction: xylanase |
First extraction: 2.92–3.85
Second extraction: 7.5–9.85 |
First extraction: 0.48–0.59
Second extraction: 0.23–0.28 |
[88] |
| Wheat and barley straw |
Alkali and steam pretreatment + enzymatic |
1–2 wt% NaOH (steam pretreatment) + β-glucosidase and xylanase |
18–35 (Wheat)
17–47 (Barley) |
n.d. |
[89] |
| Wheat bran |
Ultrasound + Enzymatic |
Xylanase (sodium acetate buffer) |
4.25–12.88 |
n.d. |
[66] |
| Wheat bran |
Enzymatic |
Xylanase |
23.1 |
0.44 |
[90] |
| Corn fibre |
Enzymatic |
Xylanase and cellulase (sodium acetate buffer) |
30–45 |
n.d. |
[90] |
* AX extracted yield by raw material dry basis (% of Dw). ** Maximum yield achieved at optimized NaOH concentration, time, and temperature (0.5 M, 2 h, 60 °C). *** Yields were dependent on the material; yield could be influenced by pretreatments of these plant materials carried out by manufacturers. n.d.: not determined.
3.4. Enzymatic Extraction of Arabinoxylans
Enzymatic extraction of AXs with the use of endoxylanases and cellulases can be as efficient as chemical methods, with the benefit that it is more environmentally friendly and AX degradation can be better controlled. Treatment conditions influence the yield, Mw, and A/X ratio of extracted AXs. The enzymatic effect on the AX extraction yield is influenced by the enzyme source and concentration, and it depends on whether they are used alone or in combination with another enzyme (
Table 2). The combined use of endoxylanases and cellulases provides higher extraction yields of AXs
[91][92]. A more common approach is to couple an enzymatic extraction of AXs with other extraction methods, typically chemical extractions with alkali solvents and chemical treatments
[13][66][69]. The enzymatic extraction can be performed after extracting the water-soluble AXs of the raw material to maximise the yield of AXs.