Carbon capture is among the most sustainable strategies to limit carbon dioxide emissions, which account for a large share of human impact on climate change and ecosystem destruction. This growing threat calls for novel solutions to reduce emissions on an industrial level. Carbon capture by amorphous solids is among the most reasonable options as it requires less energy when compared to other techniques and has comparatively lower development and maintenance costs. In this respect, the method of carbon dioxide adsorption by solids can be used in the long-term and on an industrial scale. Furthermore, certain sorbents are reusable, which makes their use for carbon capture economically justified and acquisition of natural resources full and sustainable. Clay minerals, which are a universally available and versatile material, are amidst such sorbents. These materials are capable of interlayer and surface adsorption of carbon dioxide.
- carbon sequestration
- clay minerals
1. Structural and Property Features of Clay Minerals to Support Carbon Dioxide Capture
- Kaolin-serpentine (kaolinite, nacrite, dickite etc.);
- Pyrophyllite-talc;
- Mica;
- Vermiculite;
- Smectite (montmorillonite);
- Chlorite;
- Sepiolite-palygorskite;
- Interstratified clay minerals;
- Allophane-imogolite.
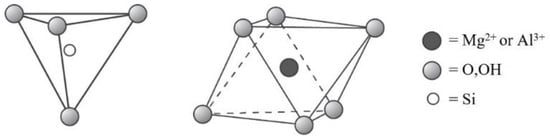
- 1:1 layer type, tetrahedral–octahedral sheet combination;
- 2:1 layer type, tetrahedral–octahedral–tetrahedral sheet combination;
- 2:1:1 layer type, tetrahedral–octahedral–tetrahedral sheet combination.
- Serpentine (e.g., chrysolite, lizardite);
- Kaolin (e.g., kaolinite, dickite, halloysite);
- Talc;
- Pyrophyllite;
- Smectite (e.g., saponite, hectorite, montmorillonite, beidellite);
- Vermiculite;
- True mica (e.g., illite, glauconite, phlogopite, biotite);
- Brittle mica (e.g., clintonite, margarite);
- Chlorite (e.g., clinochlore, chamosite, donbassite);
- Mixed layer group (e.g., chlorite-smectite, chlorite-vermiculite, illite-smectite).
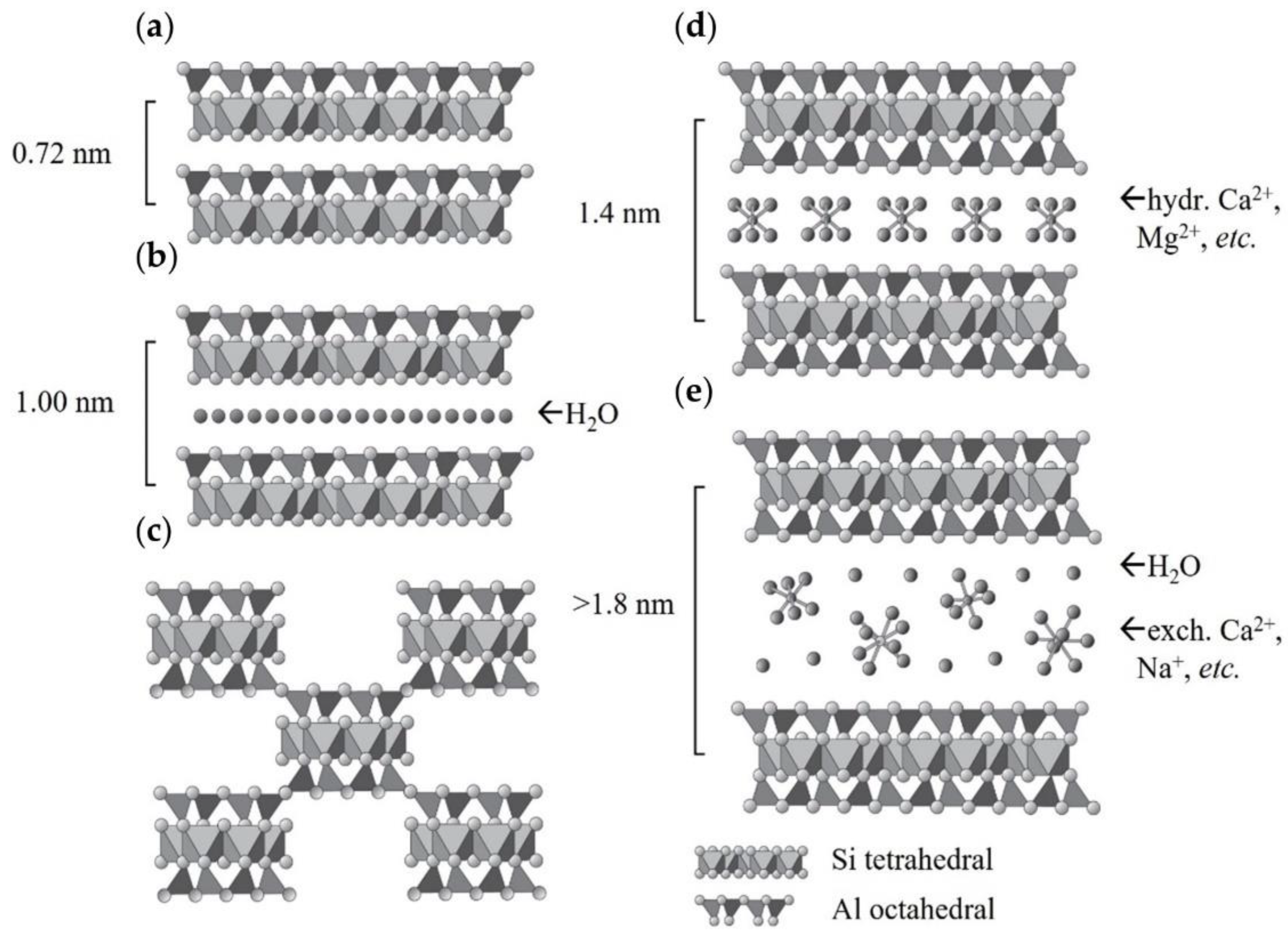
2. Clay Mineral and Carbon Dioxide Interaction Depending on Mineral Type
3. Prospective for Clay Mineral Modification to Improve Carbon Dioxide Capture
This entry is adapted from the peer-reviewed paper 10.3390/min12030349
References
- Juzkow, J. Clay Materials Modified with Amino Acids for Purification Processes of Biogas and Natural Gas. Master’s Thesis, Technical University of Lisbon, Lisbon, Portugal, 2016.
- Vaccari, A. Clays and catalysis: A promising future. Appl. Clay Sci. 1999, 14, 161–198.
- An, N.; Zhou, C.H.; Zhuang, X.Y.; Tong, D.S.; Yu, W.H. Immobilization of enzymes on clay minerals for biocatalysts and biosensors. Appl. Clay Sci. 2015, 114, 283–296.
- Guggenheim, S.; Martin, R.T. Definition of clay and clay mineral: Joint report of the AIPEA nomenclature and CMS nomenclature committees. Clays Clay Miner. 1995, 43, 255–256.
- Schulze, D.G. Clay minerals. In Encyclopaedia of Soils in the Environment; Hillel, D., Ed.; Elsevier: Boston, MA, USA, 2005; pp. 246–254.
- Theng, B.K.G. The clay minerals. Dev. Clay Sci. 2012, 4, 3–45.
- Ismadji, S.; Soetaredjo, F.E.; Ayucitra, A. Natural clay minerals as environmental cleaning agents. In Clay Materials for Environmental Remediation; Ismadji, S., Soetaredjo, F.E., Ayucitra, A., Eds.; Springer: Copenhagen, Denmark, 2015; pp. 5–37.
- Christidis, G.E. Industrial clays. In European Mineralogical Union Notes in Mineralogy. Advances in the Characterization of Industrial Clays; Christidis, G.E., Ed.; Mineralogical Society of Great Britain and Ireland: London, UK, 2010; Volume 9, pp. 341–414.
- Schoonheydt, R.A.; Johnston, C.T.; Bergaya, F. Clay minerals and their surfaces. Dev. Clay Sci. 2018, 9, 1–21.
- Nesse, W.D. Introduction to Mineralogy, 3rd ed.; Oxford University Press: New York, NY, USA, 2016.
- Clay Mineral. Available online: https://www.britannica.com/science/clay-mineral (accessed on 7 October 2021).
- Zhou, C.H.; Keeling, J. Fundamental and applied research on clay minerals: From climate and environment to nanotechnology. App. Clay Sci. 2013, 74, 3–9.
- Brigatti, M.F.; Galan, E.; Theng, B.K.H. Structures and mineralogy of clay minerals. Dev. Clay Sci. 2006, 1, 19–86.
- Brigatti, M.F.; Malferrari, D.; Laurora, A.; Elmi, C.; Mottana, A. Structure and mineralogy of layer silicates: Recent perspectives and new trends. Layered mineral structures and their application in advanced technologies. EMU Notes Mineral. 2011, 11, 1–71.
- Martin, R.T.; Bailey, S.W.; Eberl, D.D.; Fanning, D.S.; Guggenheim, S.; Kodama, H.; Pevear, D.R.; Srodon, J.; Wicks, F.J. Report of the Clay Minerals Society Nomenclature Committee: Revised classification of clay materials. Clays Clay Miner. 1991, 39, 333–335.
- Guggenheim, S.; Adams, J.M.; Bain, D.C.; Bergaya, F.; Brigatti, M.F.; Drits, V.A.; Formoso, M.L.L.; Galán, E.; Kogure, T.; Stanjek, H. Summary of recommendations of Nomenclature Committees relevant to clay mineralogy: Report of the association Internationale pour l’Etude des Argiles (AIPEA) Nomenclature Committee for 2006. Clays Clay Miner. 2006, 54, 761–772.
- Schroeder, P. Clays in the Critical Zone; Cambridge University Press: Cambridge, UK, 2018; pp. 1–246.
- Bergaya, F.; Lagaly, G. General introduction: Clays, clay minerals, and clay science. Dev. Clay. Sci. 2006, 1, 1–18.
- Michels, L.; Fossum, J.O.; Rozynek, Z.; Hemmen, H.; Rustenberg, K.; Sobas, P.A.; Kalantzopoulos, G.N.; Knudsen, K.D.; Janek, M.; Plivelic, T.S.; et al. Intercalation and retention of carbon dioxide in a smectite clay promoted by interlayer cations. Sci. Rep. 2015, 5, 8775.
- Boulet, P.; Greenwell, H.C.; Stackhouse, S.; Coveney, P.V. Recent advances in understanding the structure and reactivity of clays using electronic structure calculations. J. Mol. Struc. Theochem 2006, 762, 33–48.
- da Silva, G.J.; Fossum, J.O.; DiMasi, E.; Maloy, K.J.; Lutnaes, S.B. Synchrotron x-ray scattering studies of water intercalation in a layered synthetic silicate. Phys. Rev. E 2002, 66, 011303.
- Bordallo, H.N.; Aldridge, L.P.; Churchman, G.J.; Gates, W.P.; Telling, M.T.F.; Kiefer, K.; Fouquet, P.; Seydel, T.; Kimber, S.A.J. Quasi-elastic neutron scattering studies on clay interlayer-space highlighting the effect of the cation in confined water dynamics. J. Phys. Chem. C. 2008, 112, 13982–13991.
- Hansen, E.L.; Hemmen, H.; Fonseca, D.M.; Coutant, C.; Knudsen, K.D.; Plivelic, T.S.; Bonn, D.; Fossum, J.O. Swelling transition of a clay induced by heating. Sci. Rep. 2012, 2, 618.
- Murray, H.H. Applied Clay Mineralogy: Occurrences, Processing and Application of Kaolins, Bentonites, Palygorskite-Sepiolite, and Common Clays, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2006; pp. 1–180.
- Chouikhi, N.; Cecilia, J.A.; Vilarrasa-Garcia, E.; Besghaier, S.; Chlendi, M.; Duro, F.I.F.; Castellon, R.R.; Bagane, M. CO2 adsorption of materials synthesized from clay minerals: A review. Minerals 2019, 9, 514.
- Yariv, S.; Cross, H. Organo-Clay Complexes and Interactions; Marcel Dekker: New York, NY, USA, 2002; pp. 1–688.
- Stinkule, A. Clays in the Subterranean of Latvia; RTU Publishing House: Riga, Latvia, 2014; pp. 1–121.
- Lan, Y.; Liu, Y.; Li, J.; Chen, D.; He, G.; Parkin, I.P. Natural clay-based materials for energy storage and conversion applications. Adv. Sci. 2021, 8, 2004036.
- Cole, D.R.; Chialvo, A.A.; Rother, G.; Vlcek, L.; Cummings, P.T. Supercritical fluid behaviour at nanoscale interfaces: Implications for CO2 sequestration in geologic formations. Phil. Mag. 2010, 90, 2339–2363.
- de Jong, S.M.; Spiers, C.J.; Busch, A. Development of swelling strain in smectite clays through exposure to carbon dioxide. Int. J. Greenh. Gas Control 2014, 24, 149–161.
- Chen, Y.; Lu, D. CO2 capture by kaolinite and its adsorption mechanism. Appl. Clay Sci. 2015, 104, 221–228.
- Soares, J.L.; Moreira, R.F.P.M.; Jose, H.J.; Grande, C.A.; Rodrigues, A.E. Hydrotalcite materials for carbon dioxide adsorption at high temperatures: Characterization and diffusivity measurements. Sep. Sci. Technol. 2004, 39, 1989–2010.
- Birbara, P.J.; Nalette, T.A. Regenerable Supported Amine-Polyol Sorbent. US Patent 5,376,614, 27 December 1994.
- Yong, Z.; Rodrigues, A.E. Hydrotalcite-like compounds as adsorbents for carbon dioxide. Energy Convers. Manag. 2002, 43, 1865–1876.
- Gray, M.L.; Soong, Y.; Champagne, K.J.; Baltrus, J.; Stevens, R.W.; Toochinda, P.; Chuang, S.S.C. CO2 capture by amine-enriched fly ash carbon sorbents. Sep. Purif. Technol. 2004, 35, 31–36.
- Santos-Costa, A.; Imae, T.; Takagi, K.; Kikuta, K. Intercalation of dendrimers in the interlayer of hydrotalcite clay sheets. In Surface and Colloid Science; Springer: Berlin/Heidelberg, Germany, 2004; Volume 128, pp. 113–119.
- Sirwardane, R.V. Solid sorbents for removal of carbon dioxide from gas streams at low temperatures. US Patent 6908497, 21 June 2005.
- Chaffee, A.L.; Knowles, G.P.; Liang, Z.; Zhang, J.; Xiao, P.; Webley, P.A. CO2 capture by adsorption: Materials and process development. Int. J. Greenh. Gas Control 2007, 1, 11–18.
- Thomas, J.; Bohor, B.F. Surface area of vermiculite with nitrogen and carbon dioxide as adsorbates. Clays Clay Miner. 1969, 17, 205–209.
- Aylmore, L.A.G. Gas sorption in clay mineral systems. Clays Clay Miner. 1974, 22, 175–183.
- Azzouz, A. Physicochimie des Tamis Moléculaires; Office des Publications Universitaires: Algiers, France, 1994.
- Rodlert, M.; Plummer, C.J.G.; Leterrier, Y.; Manson, J.-A.E.; Grunbauer, H.J.M. Rheological behaviour of hyperbranched polymer/montmorillonite clay nanocomposites. J. Rheol. 2004, 48, 1049–1065.
- Lingaiah, S.; Shivakumar, K.N.; Sadler, R.; Sharpe, M. A method of visualization of dispersion of nanoplatelets in nanocomposites. Compos. Sci. Technol. 2005, 65, 2276–2280.
- Azzouz, A.; Nistor, D.; Miron, D.; Ursu, A.-V.; Sajin, T.; Monette, F.; Niquette, P.; Hausler, R. Assessment of the acid–base strength distribution of ion-exchanged montmorillonites through NH3 and CO2-TPD measurements. Thermochim. Acta 2006, 449, 27–34.
- Liu, P. Hyperbranched aliphatic polyester grafted attapulgite via a melt polycondensation process. Appl. Clay Sci. 2007, 35, 11–16.
- Azzouz, A.; Assaad, E.; Ursu, A.-V.; Sajin, T.; Nistor, D.; Roy, R. Carbon dioxide retention over montmorillonite-dendrimer materials. Appl. Clay Sci. 2010, 48, 133–137.
- Irani, M.; Fan, M.; Ismail, H.; Tuwati, A.; Dutcher, B.; Russell, A.G. Modified nanosepiolite as an inexpensive support of tetraethylenepentamine for CO2 sorption. Nano Energy 2015, 15, 235–246.
- Yuan, M.; Gao, G.; Hu, X.; Luo, X.; Huang, Y.; Jin, B.; Liang, Z. Pre-modified sepiolite functionalized with triethylenetetramine as an effective and inexpensive adsorbent for CO2 capture. Ind. Eng. Chem. Res. 2018, 57, 6189–6200.
- Thomas, J.; Bohor, B.F. Surface area of montmorillonite from the dynamic sorption of nitrogen and carbon dioxide. Clays Clay Miner. 1968, 16, 83–91.
- Fripiat, J.J.; Cruz, M.I.; Bohor, B.F.; Thomas, J. Interlamellar adsorption of carbon dioxide by smectites. Clays Clay Miner. 1974, 22, 23–30.
- Wang, Q.; Huang, L. Molecular insight into competitive adsorption of methane and carbon dioxide in montmorillonite: Effect of clay structure and water content. Fuel 2019, 239, 32–43.
- Chan, W.H.; Mazlee, M.N.; Ahmad, Z.A.; Ishak, M.A.M.; Shamsul, J.B. The development of low-cost adsorbents from clay and waste materials: A review. J. Mater. Cycles Waste Manag. 2017, 19, 293–301.
- Jeon, P.R.; Choi, J.; Yun, T.S.; Lee, C.H. Sorption equilibrium and kinetics of CO2 on clay minerals from subcritical to supercritical conditions: CO2 sequestration at nanoscale interfaces. Chem. Eng. J. 2014, 255, 705–715.
- Giesting, P.; Guggenheim, S.; Koster van Groos, A.F.; Busch, A. Interaction of carbon dioxide with Na-exchanged montmorillonite at pressures to 640 bars: Implications for CO2 sequestration. Int. J. Greenh. Gas Control 2012, 8, 73–81.
- Schaef, H.T.; Ilton, E.S.; Qafoku, O.; Martin, P.F.; Felmy, A.R.; Rosso, K.M. In situ XRD study of Ca2+ saturated montmorillonite (STX-1) exposed to anhydrous and wet supercritical carbon dioxide. Int. J. Greenh. Gas Control 2012, 6, 220–229.
- Rother, G.; Ilton, E.S.; Wallacher, D.; HauB, T.; Schaef, H.T.; Qafoku, O.; Rosso, K.M.; Felmy, A.R.; Krukowski, E.G.; Stack, A.G.; et al. CO2 sorption to sub-single hydration layer montmorillonite clay studied by excess sorption and neutron diffraction measurements. Env. Sci. Technol. 2013, 47, 205–211.
- Busch, A.; Alles, S.; Gensterblum, Y.; Prinz, D.; Dewhurst, D.N.; Raven, M.D.; Stanjek, H.; Krooss, B.M. Carbon dioxide storage potential of shales. Int. J. Greenh. Gas Control 2008, 2, 297–308.
- Maruthi Sena, M.; Krishnan, M. Role of cations in adsorption of supercritical carbon dioxide at smectite mineral-water interfaces: Molecular dynamics and adaptive biasing fore simulation studies. J. Phys. Chem. C. 2018, 123, 1170–1184.
- Narayanan Nair, A.K.; Cui, R.; Sun, S. Overview of the adsorption and transport properties of water, ions, carbon dioxide, and methane in swelling clays. ACS Earth Space Chem. 2021, 5, 2599–2611.
- Mendel, N.; Sîretanu, D.; Sîretanu, I.; Brilman, D.W.; Mugele, F. Interlayer Cation-Controlled Adsorption of Carbon Dioxide in Anhydrous Montmorillonite Clay. J. Phys. Chem. C 2021, 125, 27159–27169.
- Cecilia, J.A.; Vilarrasa-García, E.; Cavalcante, C.L.; Azevedo, D.C.S.; Franco, F.; Rodríguez-Castellón, E. Evaluation of two fibrous clay minerals (sepiolite and palygorskite) for CO2 Capture. J. Environ. Chem. Eng. 2018, 6, 4573–4587.
- Sato, K.; Hunger, M. Carbon dioxide adsorption in open nanospaces formed by overlap of saponite clay nanosheets. Comm. Chem. 2020, 3, 91.
- Azzouz, A.; Nousir, S.; Platon, N.; Ghomari, K.; Shiao, T.C.; Hersant, G.; Bergron, J.-Y.; Roy, R. Truly reversible capture of CO2 by montmorillonite intercalated with soya oil-derived polyglycerols. Int. J. Greenh. Gas Control 2013, 17, 140–147.
- Delgado, J.A.; Uguina, M.A.; Sotelo, J.L.; Ruı’z, B.; Rosa’rio, M. Carbon dioxide/methane separation by adsorption on sepiolite. J. Nat. Gas. Chem. 2007, 16, 235–243.
- Pires, J.; Bestilleiro, M.; Pinto, M.; Gil, A. Selective adsorption of carbon dioxide, methane, and ethane by porous clays heterostructures. Sep. Purif. Technol. 2008, 61, 161–167.
- Franco, F.; Cecilia, J.A.; Pozo, M.; Pardo, L.; Bellido, E.; Garcia-Sancho, C. Microwave assisted acid treatment of kerolitic clays from the Neogene Madrid Basin (Spain) and its use in CO2 capture processes. Microporous Mesoporous Mater. 2020, 292, 109749.
- Dazas, B.; Lanson, B.; Breu, J.; Robert, J.-L.; Pelletier, M.; Ferrage, E. Smectite fluorination and its impact on interlayer water content and structure: A way to fine tune the hydrophilicity of clay surfaces? Microporous Mesoporous Mater. 2013, 181, 233–247.
- da Silva, G.J.; Fossum, J.O.; DiMasi, E.; Maloy, K.J. Hydration transitions in a nanolayered synthetic silicate: A synchrotron x-ray scattering study. Phys. Rev. B 2003, 67, 094114.
- Jansson, M.; Eriksen, T.E. In situ anion diffusion experiments using radiotracers. J. Contam. Hydrol. 2004, 68, 183–192.
- Tambach, T.J.; Hensen, E.J.M.; Smit, B. Molecular simulations of swelling clay minerals. J. Phys. Chem. B 2004, 108, 7586–7596.
- Malikova, N.; Cadene, A.; Dubois, E.; Marry, C.; Durand-Vidal, S.; Turq, P.; Breu, J.; Longeville, S.; Zanotti, J.-M. Water diffusion in a synthetic hectorite clay studied by quasi-elastic neutron scattering. J. Phys. Chem. C 2007, 111, 17603–17611.
- Porion, P.; Michot, L.J.; Faugere, A.M.; Delville, A. Structural and dynamical properties of the water molecules confined in dense clay sediments: A study combining 2H NMR spectroscopy and multiscale numerical modelling. J. Phys. Chem. C 2007, 111, 5441–5453.
- Tenorio, R.P.; Alme, L.R.; Engelsberg, M.; Fossum, J.O.; Hallwass, F. Geometry and dynamics of intercalated water in Na-fluorhectorite clay hydrates. J. Phys. Chem. C 2008, 112, 575–580.
- Tenorio, R.P.; Engelsberg, M.; Fossum, J.O.; da Silva, G.J. Intercalated water in synthetic fluorhectorite clay. Langmuir 2010, 26, 9703–9709.
- Jimenez-Ruiz, M.; Ferrage, E.; Delville, A.; Michot, L.J. Anisotropy on the collective dynamics of water confined in swelling clay minerals. J. Phys. Chem. A 2012, 116, 2379–2387.
- Kloproge, J.T. Synthesis of smectites and porous pillared clay catalysts: A review. J. Porous Mater. 1998, 5, 5–41.
- Franco, F.; Pozo, M.; Cecilia, J.A.; Benitez-Guerrero, M.; Pozo, E.; Rubi, J.A.M. Microwave assisted acid treatment of sepiolite: The role of composition and “crystallinity”. Appl. Clay Sci. 2014, 102, 15–27.
- Silva, V.C.; Araujo, M.E.B.; Rodrigues, A.M.; Cartazo, J.M.; Menezes, R.R.; Neves, G.A. adsorption behaviour of acid-treated Brazilian palygorskite for cationic and anionic dyes removal from water. Sustainability 2021, 13, 3954.
- Wal, K.; Rutkowski, P.; Stawinski, W. Application of clay minerals and their derivatives in adsorption from gaseous phase. Appl. Clay Sci. 2021, 215, 106323.
- Gomez-Pozuelo, G.; Sanz-Perez, E.S.; Arencibia, A.; Pizarro, P.; Sanz, R.; Serrano, D.P. CO2 adsorption on amine-functionalized clays. Microporous Mesoporous Mater. 2019, 282, 38–47.
- Chen, Y.H.; Lu, D.L. Amine modification on kaolinites to enhance CO2 adsorption. J. Colloid Interface Sci. 2014, 436, 47–51.
- Horri, N.; Sanz-Pérez, E.S.; Arencibia, A.; Sanz, R.; Frini-Srasra, N. Amine grafting of acid-activated bentonite for carbon dioxide capture. Appl. Clay Sci. 2019, 180, 105195.
- Ramadass, K.; Sathish, C.I.; Singh, G.; Ruban, S.J.; Ruban, A.M.; Bahadur, R.; Vinu, A. Morphologically tuneable nano architectonics of mixed kaolin-halloysite derived nitrogen-doped activated nanoporous carbons for supercapacitor and CO2 capture applications. Carbon 2022, 192, 133–144.
- Bo Hunvik, K.W.; Loch, P.; Wallacher, D.; Kirch, A.; Cavalcanti, L.P.; Riess, M.; Fossum, J.O. CO2 adsorption enhanced by tuning the layer charge in a clay mineral. Langmuir 2021, 37, 14491–14499.
- Niu, M.; Yang, H.; Zhang, X.; Wang, Y.; Tang, A. Amine-impregnated mesoporous silica nanotube as an emerging nanocomposite for CO2 capture. ACS Appl. Mater. Interface 2016, 8, 17312–17320.
- Roth, E.A.; Agarwal, S.; Gupta, R.K. Nanoclay-based solid sorbents for CO2. Energy Fuels 2013, 27, 4129–4136.
- Pinto, M.L.; Mafra, L.; Guil, J.M.; Pires, J.; Rocha, J. Adsorption and activation of CO2 by amine-modified nanoporous materials studied by solid-state NMR and 13CO2 adsorption. Chem. Mater. 2011, 23, 1387–1395.
- Pinto, M.L.; Pires, J. Porous and hybrid clay-based materials for separation of hydrocarbons. Microporous Mesoporous Mater. 2012, 151, 403–410.
- Garea, S.A.; Mihai, A.J.; Ghebaur, A.; Nistor, C.; Sarbu, A. Porous clay heterostructures: A new inorganic host for 5-fluorouracil encapsulation. Int. J. Pharm. 2015, 492, 299–309.
- Chmielarz, L.; Piwowarska, Z.; Kustrowski, P.; Wegrzyn, A.; Gil, B.; Kowalczyk, A.; Dudek, B.; Dziembaj, R.; Michalik, M. Comparison study of titania pillared interlayered clay and porous clay heterostructures modified with copper and iron as catalysts of the DeNOx process. Appl. Clay Sci. 2011, 53, 164–173.
- Cecilia, J.A.; Arango-Diaz, A.; Jimenez-Jimenez, J.; Storaro, L.; Moretti, E.; Rodriguez-Castellon, E. CuO-CeO2 supported on montmorillonite-derived porous clay heterostructures (PCH) for preferential CO oxidation in H2-rich stream. Catal. Today 2015, 253, 126–136.
- Soriano, M.D.; Cecilia, J.A.; Natoli, A.; Jimenez-Jimenez, J.; Nieto, J.M.L.; Rodriguez-Castellon, E. Vanadium oxide supported on porous clay heterostructure for the partial oxidation of hydrogen sulphide to sulphur. Catal. Today 2015, 254, 36–42.
- Saboya, R.M.A.; Cecilia, J.A.; Garcia-Sancho, C.; Luna, F.M.T.; Rodriguez-Castellon, E.; Cavalcante, C.L. WO3-based catalysts supported on porous clay heterostructures (PCH) with Si–Zr pillars for synthetic esters production. Appl. Clay Sci. 2016, 124–125, 69–78.
- Azzouz, A.; Platon, N.; Nousir, S.; Ghomari, K.; Nistor, D.; Shiao, T.C.; Roy, R. OH-enriched organo montmorillonites for potential applications in carbon dioxide separation and concentration. Sep. Purif. Technol. 2013, 108, 181–188.
- Cavenati, S.; Grande, C.A.; Rodrigues, A.E. Separation of CH4/CO2/N2 mixtures by layered pressure swing adsorption for upgrade of natural gas. Chem. Eng. Sci. 2006, 61, 3893–3906.
- Grande, C.A.; Rodrigues, A.E. Electric swing adsorption for CO2 removal from flue gas. Int. J. Greenh. Gas Control 2008, 2, 194–202.
- Zhang, J.; Webley, P.A.; Xiao, P. Effect of process parameters on power requirements of vacuum swing adsorption technology for CO2 capture from flue gas. Energy Convers. Manag. 2008, 49, 346–356.
- Gupta, S.K.; Lesslie, R.D.; King, A.D. Solubility of alcohols in compressed gases. A comparison of vapor-phase interactions of alcohols and homomorphic compounds with various gases I. Ethanol in compressed helium, hydrogen, argon, methane, ethylene, ethane, carbon dioxide, and nitrous oxide. J. Phys. Chem. 1973, 77, 2011–2015.
- Massoudi, R.; King, A.D. Solubility of alcohols in compressed gases. A comparison of vapor-phase interactions of alcohols and homomorphic compounds with various gases II. 1-Butanol, diethyl ether, and n-pentane in compressed nitrogen, argon, methane, ethane, and carbon dioxide at 25.deg. J. Phys. Chem. 1973, 77, 2016–2018.
- Saharay, M.; Balasubramanian, S. Electron donor–acceptor interactions in ethanol—CO2 mixtures: An ab initio molecular dynamics study of supercritical carbon dioxide. J. Phys. Chem. B 2006, 110, 3782–3790.
- Azzouz, A.; Ursu, A.-V.; Nistor, D.; Sajin, T.; Assaad, E.; Roy, R. TPD study of the reversible retention of carbon dioxide over montmorillonite intercalated with polyol dendrimers. Thermochim. Acta 2009, 496, 45–49.
- Calderon, M.; Quadir, M.A.; Sharma, S.K.; Haag, R. Dendritic polyglycerols for biomedical applications. Adv. Mater. 2010, 22, 190–218.
- Gassensmith, J.J.; Furukawa, H.; Smaldone, R.A.; Forgan, R.S.; Botros, Y.Y.; Yaghi, O.M.; Stoddart, J.F. Strong and reversible binding of carbon dioxide in a green metal–organic framework. J. Am. Chem. Soc. 2011, 133, 15312–15315.
- Garcia-Gallastegui, A.; Iruretagoyena, D.; Gouvea, V.; Mokhtar, M.; Asiri, A.M.; Basahel, S.N.; Al-Thabaiti, S.A.; Alyoubi, A.O.; Chadwick, D.; Sha, M.S.P. Graphene oxide as support for layered double hydroxides: Enhancing the CO2 adsorption capacity. Chem. Mater. 2012, 24, 4531–4539.
- Galarneau, A.; Barodawalla, A.; Pinnavaia, T.J. Porous clay heterostructures formed by gallery-templated synthesis. Nature 1995, 374, 529–531.
- Cecilia, J.A.; Garcia-Sancho, C.; Franco, F. Montmorillonite based porous clay heterostructures: Influence of Zr in the structure and acidic properties. Microporous Mesoporous Mater. 2013, 176, 95–102.
- Humphrey, J.; Boyd, D. Clay: Types, Properties and Uses; Nova Science Publishers, Inc.: New York, NY, USA, 2011.
- Nawani, P.; Gelfer, M.Y.; Hsiao, B.S.; Frenkel, A.; Gilman, J.W.; Khalid, S. Surface Modification of nanoclays by catalytically active transition metal Ions. Langmuir 2007, 23, 9808–9815.
- Zope, I.S.; Dasari, A.; Camino, G. Elucidating the catalytic effect of metal ions in montmorillonite on thermal degradation of organic modifier. Mater. Chem. Phys. 2015, 157, 69–79.
- Reddy, C.R.; Bhat, Y.S.; Nagendrappa, G.; Jai Prakash, B.S. Brønsted and Lewis acidity of modified montmorillonite clay catalysts determined by FT-IR spectroscopy. Catal. Today 2009, 141, 157–160.
- Tang, T.; Chen, X.; Meng, X.; Chen, H.; Ding, Y. Synthesis of multiwalled carbon nanotubes by catalytic combustion of polypropylene. Angew. Chem. Int. Ed. 2005, 44, 1517–1520.
- Tang, T.; Chen, X.; Chen, H.; Meng, X.; Jiang, Z.; Bi, W. Catalyzing carbonization of polypropylene itself by supported nickel catalyst during combustion of polypropylene/clay nanocomposite for improving fire retardancy. Chem. Mater. 2005, 17, 2799–2802.
- Hu, Y.; Wang, X.; Li, J. Regulating effect of exfoliated clay on intumescent char structure and flame retardancy of polypropylene composites. Ind. Eng. Chem. Res. 2016, 55, 5892–5901.
