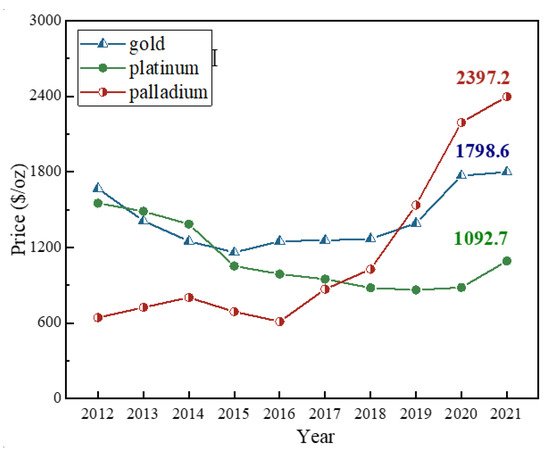
The spent automobile catalysts (SAC) is the major secondary source of palladium and the production of SAC is increasing rapidly over years. The price of palladium keeps rising over the years, which demonstrates its preciousness and urgent industrial demand. Recovering palladium from the spent automobile catalysts benefits a lot from economic and environmental protection aspects. Hydrometallurgical methods such as chloride leaching with oxidants possess a high selectivity of palladium and low consumption of energy, and are cost-effective and flexible for different volume feeds compared with pyrometallurgical methods. The recovery ratios of palladium and other platinum-group metals should be the focus of competition since their prices have been rapidly increased over the years, and hence more efficient extractants with high selectivity of palladium even in the complexed leachate should be proposed in the future.
- spent automobile catalysts
- palladium recovery
- hydrometallurgy
- platinum group metals
- palladium leaching
1. Introduction
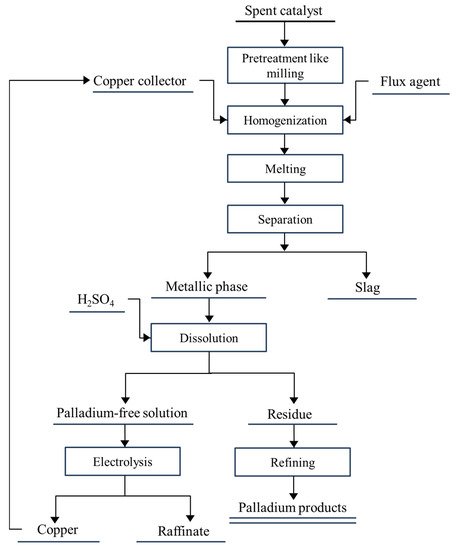
2. The Development of Recovering Palladium from the Spent Automobile Catalysts
2.1. Pretreatment
2.2. Hydrometallurgy
2.2.1. Leaching or Extraction
2.2.2. Separation or Recovery of Palladium from Leachate
Precipitation
Solvent Extraction
Ion Exchange
2.3. Pyrometallurgy
2.3.1. Chlorination Volatilization Method
2.3.2. Metal Capture Method
This entry is adapted from the peer-reviewed paper 10.3390/met12040533
References
- Jha, M.K.; Lee, J.C.; Kim, M.S.; Jeong, J.; Kim, B.S.; Kumar, V. Hydrometallurgical recovery/recycling of platinum by the leaching of spent catalysts: A review. Hydrometallurgy 2013, 133, 23–32.
- Ding, Y.J.; Zhang, S.E.; Liu, B.; Zheng, H.D.; Chang, C.C.; Ekberg, C. Recovery of precious metals from electronic waste and spent catalysts: A review. Resour. Conserv. Recycl. 2019, 141, 284–298.
- Zheng, H.; Ding, Y.; Wen, Q.; Liu, B.; Zhang, S. Separation and purification of platinum group metals from aqueous solution: Recent developments and industrial applications. Resour. Conserv. Recycl. 2021, 167, 105417–105433.
- Yakoumis, I.; Panou, M.; Moschovi, A.M.; Panias, D. Recovery of platinum group metals from spent automotive catalysts: A review. Clean. Eng. Technol. 2021, 3, 100112–100122.
- Dong, H.; Zhao, J.; Chen, J.; Wu, Y.; Li, B. Recovery of platinum group metals from spent catalysts: A review. Int. J. Miner. Processing 2015, 145, 108–113.
- Xu, A.Y.; Ye, T.; Zhao, S.H. Recovery of Valuable Metals from Spent Hydrogenation Catalysts. In Advanced Materials & Sports Equipment Design; Applied Mechanics and Materials; Yang, D., Zhang, T.B., Luo, Q., Eds.; Trans Tech Publications Ltd.: Bäch SZ, Switzerland, 2014; Volume 440, pp. 97–103.
- Fajar, A.T.N.; Hanada, T.; Firmansyah, M.L.; Kubota, F.; Goto, M. Selective Separation of Platinum Group Metals via Sequential Transport through Polymer Inclusion Membranes Containing an Ionic Liquid Carrier. ACS Sustain. Chem. Eng. 2020, 8, 11283–11291.
- Yu, W.L.; Chen, Z.; Yu, S.T.; Ding, J.W.; Shan, Y.L.; Liu, F.S.; Li, M. Highly dispersed Pt catalyst supported on nanoporous carbon derived from waste PET bottles for reductive alkylation. Rsc. Adv. 2019, 9, 31092–31101.
- Barakat, M.A.; Mahmoud, M.H.H.; Mahrous, Y.S. Recovery and separation of palladium from spent catalyst. Appl. Catal. A-Gen. 2006, 301, 182–186.
- KITCO. Available online: https://www.kitco.com/charts/livepalladium.html (accessed on 15 January 2022).
- Peng, Z.; Li, Z.; Lin, X.; Tang, H.; Ye, L.; Ma, Y.; Rao, M.; Zhang, Y.; Li, G.; Jiang, T. Pyrometallurgical Recovery of Platinum Group Metals from Spent Catalysts. JOM 2017, 69, 1047–4838.
- Alekseeva, T.Y.; Karpov, Y.A.; Dal‘nova, O.A.; Es‘kina, V.V.; Baranovskaya, V.B.; Gorbatova, L.D. Current State and Problems of Analytical Control of Spent Automobile Catalysts (Review). Inorg. Mater. 2018, 54, 1421–1429.
- Rzelewska, M.; Regel-Rosocka, M. Wastes generated by automotive industry—Spent automotive catalysts. Phys. Sci. Rev. 2018, 3, 2365–2381.
- Al-Sheeha, H.; Marafi, M.; Raghavan, V.; Rana, M.S. Recycling and Recovery Routes for Spent Hydroprocessing Catalyst Waste. Ind. Eng. Chem. Res. 2013, 52, 12794–12801.
- A Threefold Increase in Two Years, the Myth of Palladiun. Available online: https://news.smm.cn/news/100877360. (accessed on 15 January 2022).
- Chauhan, G.; Pant, K.K.; Nigam, K.D.P. Metal Recovery from Hydroprocessing Spent Catalyst: A Green Chemical Engineering Approach. Ind. Eng. Chem. Res. 2013, 52, 16724–16736.
- Zhang, L.; Song, Q.; Liu, Y.; Xu, Z. Novel approach for recovery of palladium in spent catalyst from automobile by a capture technology of eutectic copper. J. Clean. Prod. 2019, 239, 50–59.
- Jimenez de Aberasturi, D.; Pinedo, R.; Ruiz de Larramendi, I.; Ruiz de Larramendi, J.I.; Rojo, T. Recovery by hydrometallurgical extraction of the platinum-group metals from car catalytic converters. Miner. Eng. 2011, 24, 505–513.
- Angelidis, T.N. Development of a laboratory scale hydrometallurgical procedure for the recovery of Pt and Rh from spent automotive catalysts. Top. Catal. 2001, 16, 419–423.
- Wang, J.; Zhong, Z.P.; Song, Z.W.; Ding, K.; Deng, A.D. Modification and regeneration of HZSM-5 catalyst in microwave assisted catalytic fast pyrolysis of mushroom waste. Energy Convers. Manag. 2016, 123, 29–34.
- Suoranta, T.; Zugazua, O.; Niemelä, M.; Perämäki, P. Recovery of palladium, platinum, rhodium and ruthenium from catalyst materials using microwave-assisted leaching and cloud point extraction. Hydrometallurgy 2015, 154, 56–62.
- Lin, G.; Cheng, S.; Wang, S.X.; Hu, T.; Peng, J.H.; Xia, H.Y.; Jiang, F.; Li, S.W.; Zhang, L.B. Process optimization of spent catalyst regeneration under microwave and ultrasonic spray-assisted. Catal. Today 2018, 318, 191–198.
- Ye, X.L.; Koppala, S.; Qu, W.W.; Xu, S.M.; Zhang, L.B.; Liu, B.G.; Guo, S.H.; Wang, L. New approach to the utilization of microwave thermal energy: Desulfurization and decarburization of spent catalyst via microwave treatment. Powder Technol. 2018, 338, 764–773.
- Spooren, J.; Atia, T.A. Combined microwave assisted roasting and leaching to recover platinum group metals from spent automotive catalysts. Miner. Eng. 2020, 146, 0892–0895.
- Jun, R. Recovery of spent catalysts. Rare Met. Separately 1994, 118, 0926–0928.
- Saily, A.; Khurana, U.; Yadav, S.K.; Tandon, S.N. Thiophosphinic acids as selective extractants for molybdenum recovery from a low grade ore and spent catalysts. Hydrometallurgy 1996, 41, 0304–0308.
- Paiva, A.P.; Ortet, O.; Carvalho, G.I.; Nogueira, C.A. Recovery of palladium from a spent industrial catalyst through leaching and solvent extraction. Hydrometallurgy 2017, 171, 394–401.
- Nogueira, C.A.; Paiva, A.P.; Oliveira, P.C.; Costa, M.C.; da Costa, A.M. Oxidative leaching process with cupric ion in hydrochloric acid media for recovery of Pd and Rh from spent catalytic converters. J. Hazard. Mater. 2014, 278, 82–90.
- Chen, J.; Huang, K. A new technique for extraction of platinum group metals by pressure cyanidation. Hydrometallurgy 2006, 82, 164–171.
- Sobral, L.G.S.; Granato, M. Palladium: Extraction and refining. Miner. Eng. 1992, 5, 17–25.
- Chao, Y. Research on Recovery of Palladium from the Spent Pd/Al2O3 Catalyst. Master’s Dissertation, Northeastern University, Shenyang, China, 2016.
- Zhang, Z.H. The separation and purification of palladium from the spent catalyst. Miner. Processing 2002, 54, 60–62.
- Singh, R.; Khwaja, A.R.; Gupta, B.; Tandon, S.N. Extraction and separation of nickel(II) using bis(2,4,4-trimethylpentyl) dithiophosphinic acid (Cyanex 301) and its recovery from spent catalyst and electroplating bath residue. Solvent Extr. Ion Exch. 1999, 17, 367–390.
- Paiva, A.P. Recycling of Palladium from Spent Catalysts Using Solvent Extraction-Some Critical Points. Metals 2017, 7, 505.
- Swain, B.; Jeong, J.; Kim, S.-K.; Lee, J.-C. Separation of platinum and palladium from chloride solution by solvent extraction using Alamine 300. Hydrometallurgy 2010, 104, 1–7.
- Traeger, J.; König, J.; Städtke, A.; Holdt, H.-J. Development of a solvent extraction system with 1,2-bis(2-methoxyethylthio)benzene for the selective separation of palladium(II) from secondary raw materials. Hydrometallurgy 2012, 127–128, 30–38.
- Lee, J.Y.; Raju, B.; Kumar, B.N.; Kumar, J.R.; Park, H.K.; Reddy, B.R. Solvent extraction separation and recovery of palladium and platinum from chloride leach liquors of spent automobile catalyst. Sep. Purif. Technol. 2010, 73, 213–218.
- Nguyen, T.H.; Sonu, C.H.; Lee, M.S. Separation of Pt(IV), Pd(II), Rh(III) and Ir(IV) from concentrated hydrochloric acid solutions by solvent extraction. Hydrometallurgy 2016, 164, 71–77.
- Costa, M.C.; Assunção, A.; Almeida, R.; da Costa, A.M.R.; Nogueira, C.; Paiva, A.P. N,N′-dimethyl-N,N′-dicyclohexylsuccinamide: A novel molecule for the separation and recovery of Pd(II) by liquid-liquid extraction. Sep. Purif. Technol. 2018, 201, 96–105.
- Sharma, S.; Rajesh, N. Augmenting the adsorption of palladium from spent catalyst using a thiazole ligand tethered on an amine functionalized polymeric resin. Chem. Eng. J. 2016, 283, 999–1008.
- Dewan, A.; Sarmah, M.; Bora, U.; Thakur, A.J. In situ generation of palladium nanoparticles using agro waste and their use as catalyst for copper-, amine- and ligand-free Sonogashira reaction. Appl. Organomet. Chem. 2017, 31, 0268–0272.
- Maeda, M.; Narita, H.; Tokoro, C.; Tanaka, M.; Motokawa, R.; Shiwaku, H.; Yaita, T. Selective extraction of Pt(IV) over Fe(III) from HCl with an amide-containing tertiary amine compound. Sep. Purif. Technol. 2017, 177, 176–181.
- Sarioglan, S. Recovery of Palladium from Spent Activated Carbon-Supported Palladium Catalysts. Platin. Met. Rev. 2013, 57, 289–296.
- Yao, Z.H. The synthesis of the amido oximime chelate fiber and its adsorption behavior of palladium. Chin. Polym. Mater. Sci. Eng. 1999, 15, 10–13.
- Gan, S.C. Separation and enrichment of Gold, platinum and palladium by dt-1016 anion exchange resin. Chin. Rock Miner. Anal. 2002, 2, 113–116.
- Turanov, A.N.; Karandashev, V.К.; Artyushin, O.I.; Sharova, E.V.; Genkina, G.K. Adsorption of palladium(II) from hydrochloric acid solutions using polymeric resins impregnated with novel N-substituted 2-(diphenylthiophosphoryl)acetamides. Sep. Purif. Technol. 2017, 187, 355–364.
- Hageluken, C. Recycling of spent noble metal catalysts with emphasis on pyrometallurgical processing. Oil Gas-Eur. Mag. 1999, 25, 36–39.
- Liu, C.; Sun, S.C.; Zhu, X.P.; Tu, G.F.; Zhang, J.Y. Recovery of platinum from the spent auto-catalysts by pyrometallurgy. In Proceedings of the 3rd International Conference on New Material and Chemical Industry, Sanya, China, 17–19 November 2018.
- Varley, T. The chloride volatilization process. J. Frankl. Inst. 1923, 195, 715–716.
- Benson, M.; Bennett, C.R.; Harry, J.E.; Patel, M.K.; Cross, M. The recovery mechanism of platinum group metals from catalytic converters in spent automotive exhaust systems. Resour. Conserv. Recycl. 2000, 31, 1–7.
- Zheng, T.; He, J.; Zhao, Y.; Xia, W.; He, J. Precious metal-support interaction in automotive exhaust catalysts. J. Rare Earths 2014, 32, 97–107.
- Zheng, H.; Ding, Y.; Wen, Q.; Zhao, S.; He, X.; Zhang, S.; Dong, C. Slag design and iron capture mechanism for recovering low-grade Pt, Pd, and Rh from leaching residue of spent auto-exhaust catalysts. Sci. Total Environ. 2022, 802, 1498–1504.
