The spent automobile catalysts (SAC) is the major secondary source of palladium and the production of SAC is increasing rapidly over years. The price of palladium keeps rising over the years, which demonstrates its preciousness and urgent industrial demand. Recovering palladium from the spent automobile catalysts benefits a lot from economic and environmental protection aspects. Hydrometallurgical methods such as chloride leaching with oxidants possess a high selectivity of palladium and low consumption of energy, and are cost-effective and flexible for different volume feeds compared with pyrometallurgical methods. The recovery ratios of palladium and other platinum-group metals should be the focus of competition since their prices have been rapidly increased over the years, and hence more efficient extractants with high selectivity of palladium even in the complexed leachate should be proposed in the future.
1. Introduction
Platinum group metals (PGMs) such as platinum (Pt), palladium (Pd), and rhodium (Rh) are widely used in automobile, chemical engineering, petroleum, electrical, and electronic industries due to their distinct physical and chemical properties: catalytic activity, electric conductivity, and corrosion resistance
[1][2][3][4][5]. The increasing demand for PGMs causes a high consumption rate of high-grade PGMs ore. The increasing depletion of high-grade PGMs ore forces people to turn to the exploitation of low-grade ore. However, the cost of exploring PGMs from low-grade ore is much expensive and brings critical environmental issues
[6][7]. Although researchers have studied fungible materials to partially replace the use of PGMs in products such as automobile catalysts, the net consumption of PGMs is still high due to the increasing demand for engines in automobiles. About 65% of palladium, 45% of platinum, and 84% of rhodium are used every year for catalytic converters to decrease harmful emissions from engines
[3][5][8][9]. With the enforcement of stricter emission regulations, the rapid development of the new-energy automobile industry will lead to a larger drain on PGMs supply. Meanwhile, PGMs in spent catalysts from numbers of outdated vehicles need to be properly recycled.
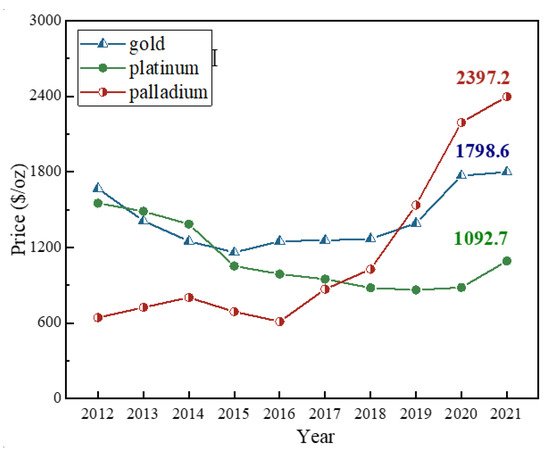
Compared with natural raw ore, the spent automobile catalysts indeed own several advantages. Palladium, like gold, is an international precious metal spot and trading species. Palladium surpassed the price of platinum for the first time in the year 2018, and then exceeded the price of gold in the year 2019
[10]. Spot palladium hit a record high at 481.7 RMB/g or 2397.2 USD/oz in 2021, while platinum was only 219.6 RMB/g or 1092.7 USD/oz and gold hovered at 361.4 RMB/g or 1798.6 USD/oz. As for rhodium, its price reached as high as 3631.8 RMB/g or 18074.0 USD/oz, which was ten times of that of gold.
[10]. The price of palladium keeps increasing at a fast speed at present as shown in
Figure 1. Palladium could partially substitute platinum in catalytic converters for most gasoline-fueled vehicles, and industries favored using palladium over platinum in catalytic converters because the price of palladium was lower than platinum decades ago, which resulted in amounts of palladium remaining in the automobile exhaust catalysts
[11][12][13][14].
The great consumption of palladium products leads to a great amount of waste and scrap containing palladium. Recovery of palladium from the spent automobile catalysts has become an important source of palladium supply
[15]. Compared with natural raw ore, the spent automobile catalysts indeed possess several advantages for palladium recovery. For one thing, the concentration of palladium in raw ore is pretty low, in the range of 2~10 ppm (g/t), and is generally associated with base metal sulfide minerals. While the spent catalysts are up to 1~9 × 10
3 ppm, that is, thousands of times higher than that of raw ore. The recovery ratios of palladium from the spent automobile catalysts with hydrometallurgical technologies, e.g., traditional cyanide leaching or chloride leaching, could reach over 95%, and through pyrometallurgy, such as iron capture, the recovery efficiency of palladium is as high as 99%
[2]. For another, the next is the carrier of spent catalysts commonly is Al
2O
3, activated carbon, and cordierite, which indicates no need to deal with ferromagnetic silicate gangue like raw material. Besides, the spent catalysts are easy to collect due to their concentrated usage, and some industries have formed completed recovery processes
[5][16]. The advantages listed above make the spent catalysts relatively easy to process, together with low capital investment, low environmental pollution, and high economic benefits. Recovery of palladium from the spent catalysts is beneficial from the perspectives of economic efficiency and environmental protection.
2. The Development of Recovering Palladium from the Spent Automobile Catalysts
With the rapid development of the automobile industry, palladium has been widely used in automobile catalysts due to its unique and excellent catalytic performance
[17]. Around half of the global palladium is used to produce automobile catalysts. However, the recycled palladium from spent catalysts is less than 25% of its application
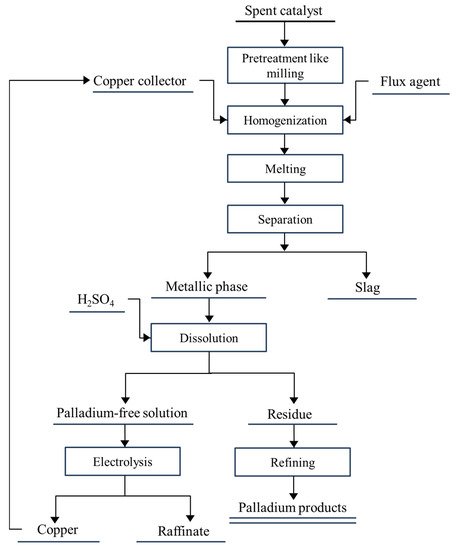
[18]. The general overflow of recovery of PGMs from the spent catalysts can be broken into several steps: homogenization, preconcentration/pretreatment, leaching/dissolution, extraction/metal isolation, and purification. Methods of recovering palladium from spent catalysts can be divided into hydrometallurgical and pyrometallurgical methods. Hydrometallurgy includes traditional cyanide leaching, thiosulfate leaching, HCl(aq) + oxidants leaching, bio-recovery, and supercritical fluid extraction; pyrometallurgy includes incineration, chloride volatilization, and metal collection. In particular, the hydrometallurgical methods are reported more frequently because of their flexibility for raw material, lower energy consumption, and mild operation environment. In this section, the researchers introduce and evaluate related studies and technologies of recovering palladium from the spent automobile catalysts.
This entry is adapted from the peer-reviewed paper 10.3390/met12040533