In the surge of the current, alarming scenario of SARS-CoV-2 infections, there is a immediate necessity for developing highly-innovative antiviral agents to work against the viruses with a broad spectrum of antiviral activity. Here-in, science-based methods, mechanisms, and procedures are implemented in obtaining resultant antiviral coated substrates, used in the destruction of the strains of the different viruses are presented. we pay particular attention to recent examples from the materials science and engineering field that highlight how some classes of antiviral drug candidates, such as polymeric materials, metal ions/metal oxides and functional nanomaterials result in acting against the viral spread. Based on the available literature and data, we also disclose some of the strategies for development of a universal and reusable virus deactivation system against the emerging COVID-19.
- COVID-19
- Antiviral coatings,
- Pandemic
- Antiviral products
1. Introduction
The presence of different microorganisms in nature may sometimes cause a detrimental impact on human health [1]. Specifically, viruses have always been regarded as increasing hazards by impairing health, as human contact with these microbes from the environment can lead to extreme illnesses and other ailments [2]. For example, tropical and subtropical countries survived an outbreak of dengue virus, known to cause the severe form of dengue hemorrhagic fever/dengue shock syndrome (DHS/DSS) [3][4]. Since the emergence of the Spanish flu outbreak (1918), influenza viral pandemics are known to appear within the interval of every 10 to 15 years [5]. Characterized by their variations in pathogenicity, the most virulent type, A influenza viruses (H1N1 and H5N1) (2009), are known to cause serious human pandemics via common transmission from animals to humans and vice versa [6][7]. Lethal varieties of coronavirus, such as severe acute respiratory syndrome-related coronavirus (SARSr-CoV) and middle east respiratory syndrome-related coronavirus (MERS-CoV) are known to cause SARS (2003) and MERS (2014) outbreaks, respectively. These coronavirus related infections were reported in several countries of North America, South America, Europe, and Asia [8][9]. Recently, an Ebola hemorrhagic fever (EHF) (2014) outbreak severely affected the living species of Africa [10][11]. In late December 2019, the emergence of a novel pneumonia drew animated attention around the world. Visualizing the chronological order, the ingenious agent that was responsible for causing the novel pneumonia has been identified as a novel coronavirus (nCoV or SARS-CoV-2) [12][13]. The outbreak of a novel coronavirus disease (COVID-19) has created a devastating challenge to the human health of various sections in the world [14]. It has caused negative social effects and massive economic damage, on a global scale. Coronaviruses contain spherical even-shaped virions, a type of enveloped RNA virus initially causing respiratory unevenness, and further leading to extreme flu [15][16]. An increased concern has arisen in the recent past with respect to a growing number of new, more virulent and pathologic viruses, such as those associated with SARS and, more recently COVID-19 [17][18].
Microorganisms constitute both bacteria and viruses [19]. Most of the antimicrobial coatings so far developed and commercialized are antibacterial, but there are very few reports on commercialized antiviral coatings. Hence, it is highly desirable to search for potential antiviral and viricidal elements (materials and coatings) to design personal protective equipment (PPE), hygienic implements, and other devices to fight against the rise of viral pandemics and virus-associated fatal risks [20]. This encyclopedia presents the techniques and methods that are involved in the design and development of different antiviral coatings, aiming to inspire strategies for development of coatings that are supposed to enhance antiviral efficiency, eliciting their potential application in the inhibition of COVID 19 like pandemics.
Members of antiviral coatings have been divided into three major groups (antiviral polymers, metal ions/metal oxides, and functional nanomaterials), based on the type of materials used at the contaminated sites. The methods for the treatment of virus affected substrates for preventing the virus deposition over the surfaces, using antiviral and viricidal coatings are discussed. The potential antiviral and viricidal coating technologies implemented, for design and development of a wide range of commercialized antiviral products, such as personal protective equipment (PPE), medical instrument, appliances, and hygienic implements, are discussed. Antiviral products are designed with the concept of modifying the surface, with any of the antiviral and viricidal coating compositions, using the most promising surface modification technologies [21][22][23][24][25]. Both antiviral and viricidal compositions and surface modification technologies play a major role in the destruction of viruses, by providing a thin film over the surface to retain its antiviral activity.
2. Coatings Empowering Antiviral and Viricidal Properties
2.1. Antiviral Polymers
Polymers present unique properties, such as impact resistance, ductility, and elasticity, which can be tailored for various biomedical applications. Currently, a wide range of polymers have been used for antiviral composite materials. Some antiviral agents can be encapsulated in polymers to form antiviral composites, which are released upon specific requirement. Polymers with antiviral and viricidal effects are mainly used as coatings, covering the surface of a wide range of substrates. Consistently, a section of polymers showcase their excellent antiviral and antibacterial effects due to their resistance to the adhesion of bacteria and viruses [26]. Figure 1 illustrates the antiviral mechanism of typical polymer coatings.
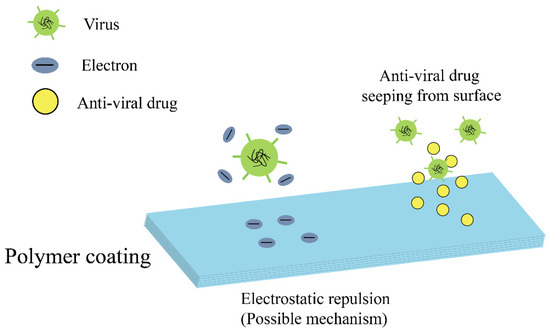
Figure 1. Schematic demonstration of the antiviral mechanism of polymer coatings.
2.2. Antiviral Metal Ions/Metal Oxides
In the past decades, a large number of metal ions and metal oxides have been investigated for their antiviral and viricidal activity, particularly silver, copper, and zinc-based ions/oxides. In general, these metal materials show lower toxicological effects and high antiviral activity compared to other metals, making them a popular fit for this kind of application. Conventionally, both the metal ions and metal oxides exhibit similar antiviral mechanisms of control over different viral strain. For instance, metal ions can adhere to the viral envelope, the membrane of cells, and then enter the interior, destroying genetic materials such as DNA and RNA [27]. The possible mechanism of action of metal ions/oxides is illustrated in Figure 2.
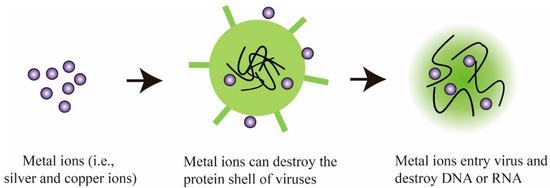
Figure 2. Schematic showing the antiviral mechanism of metal ions.
2.3. Antiviral Functional Nanomaterials
According to the nanoscale properties of viruses, it’s possible to develop certain hybrid nanomaterials with multiple functionalities, to achieve viricidal effects, since they are considered to be highly effective in controlling viral infections. Figure 3 illustrates the antiviral mechanisms of functional nanomaterials. The absolute biological effect could be determined by the physical and chemical characterization, and by functionalized chemical modification of the materials.
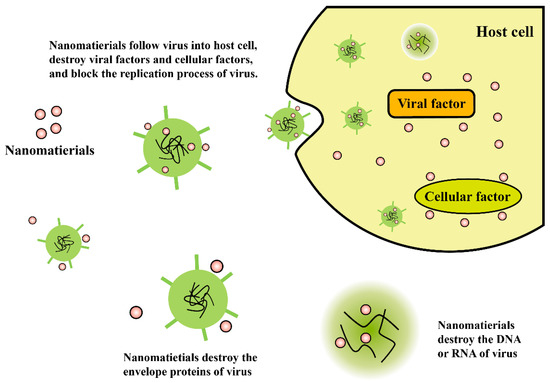
Figure 3. Illustration of antiviral mechanisms of functional nanomaterials.
3. Antiviral Products
Coating materials are continuously evolving, and their practical applications in antiviral products have great public health, and social, importance. Aside from the antiviral mechanisms presented with polymers, metal ions and functional nanomaterials, this section details about some of the other antiviral embodiments in the destruction of different viral strains and also establishes competent hypothesis behind their application as antiviral products for COVID-19. Chu et al. have demonstrated, in a systematic analysis, that wearing face masks may protect people against infection by coronaviruses [28]. This report motivates and prompts the development of various materials to manufacture the antiviral products against COVID-19. CytaCoat is the first antimicrobial coating developed with biocompatible organic components to inactivate SARS-COV-2 (COVID-19). CytaCoat is not dependent on toxic metal ions or metal oxides, hence it finds its application in face masks to prevent the spread of novel coronaviruses [29]. Another invention reports a lightweight and disposable antiviral respirator mask, which comprises of a filter that may preferably include a passive layer and an active disinfectant layer. This market available product was able to kill viruses with a small size, hardly blocked by only passive filters [30]. In another embodiment, an invention used a cartridge or filter inserted in a gas or respirator mask for destroying hazardous viral substances [31]. In the absence of vaccination for the emerging viral pandemics, respirators and masks can be worn to prevent transmission of airborne pathogenic aerosols and control the diseases such as influenza and the coronaviruses responsible for COVID-19 [32]. These devices are capable of trapping the viruses floating in the ambient air at a higher rate, followed by inactivating the viruses trapped from the air. Lightweight and breathable fiber fabrics containing antiviral and viricidal properties can be developed to replace the intermediate layers of currently available masks, to use them as personal protective clothing, with necessary antiviral and viricidal activity. These potential fabrics can be developed using antiviral polymers and a mixture of antiviral or viricidal nanoparticles and polymers. The special grade composite polymer solutions can either be coated onto the surface or can be electro-spun into the fiber fabrics to address the antiviral or viricidal activity [33][34][35]. The fluidic compositions are developed as supportive coatings with highly efficient antiviral properties. The fluid compositions in particular are premeditated to cover the articles that are plausible to carry the viruses, especially toys, health devices, notes, cards, etc. The presence of virus on theese objects may lead to contamination, while the incorporation of fluidic compositions may prevent these events. Rossett et al. proposed a fluidic composition that comprises of at least one viricide, like lauric acid and essential oils like laurel essential oil, or soya bean oil, having an antiviral activity. The ability of the viricide in the composition makes the surface look transparent, with an effective coating [36]. Fox et al. proposed a potential antiviral composition that includes a powdered substrate solution and an organic acid. This solid combination could be applied directly onto the substrate to destroy rhino and rota viruses. The composition plays a major role in the destruction of non-enveloped and enveloped influenza viruses, and also provides a thin film of organic acid on the surface to continue its antiviral activity. These antiviral compositions present some strong efficacious solutions, against a broad-spectrum of viruses, and also makes it feasible to produce at a lower cost [37].
The current limitation faced by the antiviral coatings is their usage in wet areas like sinks and faucets, due to their resistance to water. To resolve these issues, Watanabe et al. combined a particular polyhydric carboxylic acid with quaternary ammonium salt, which serves as an antiviral component used in coating material like paints and varnishes, to form highly durable antiviral, water-resistant films [30]. Torkelson et al. investigated a quaternary ammonium silane compound attached to sand filters to effectively treat bacteria and virus from drinking water. This could destroy certain strains of E.coli, Ms2 colliphage, poliovirus type3, and Adenovirus type2, thus demonstrating its application in both antimicrobial and antiviral water filters [38]. These antiviral compositions provide the advantages of a broad-spectrum of viral control. Continuous antiviral activity is not only maintained due to the presence of these compositions, but also this activity is sustained because a residual barrier film of composition ingredients is incorporated onto the objects/surfaces.
4. Conclusion
In this encyclopedia, specific coating materials that inactivate viruses have been discussed. Various strategies involved in the development of antiviral and viricidal coatings, like modifying the surface of a substrate via antiviral polymers, incorporation of metal ions/oxides, and functional nanoparticles were discussed. The antiviral efficacies of the developed coatings were detailed, and their possible and promising applications were further correlated with emerging viral pandemics, like COVID-19. We attempt to summarize and improvise the extension of present techniques, with certain modifications, for the prevention of COVID-19, and further inspire the future antiviral strategies.
This entry is adapted from the peer-reviewed paper 10.3390/ma13184041
References
- Howell, A.B.; D'Souza, D.H. The Pomegranate: Effects on Bacteria and Viruses That Influence Human Health. Evidence-Based Complementary and Alternative Medicine 2013, 2013, 606212, doi:10.1155/2013/606212.
- Daszak, P.; Cunningham, A.A.; Hyatt, A.D. Emerging Infectious Diseases of Wildlife-- Threats to Biodiversity and Human Health. Science 2000, 287, 443, doi:10.1126/science.287.5452.443.
- Rajapakse, S.J.J.o.E., Trauma; Shock. Dengue shock. 2011, 4, 120.
- Anderson, L.J.; Parker, R.A.; Strilms, R.L.J.J.o.I.D. Association between respiratory syncytial virus outbreaks and lower respiratory tract deaths of infants and young children. The Journal of Infectious Diseases 1990, 161, 640-646, doi.org/10.1093/infdis/161.4.640.
- Hsieh, Y.-C.; Wu, T.-Z.; Liu, D.-P.; Shao, P.-L.; Chang, L.-Y.; Lu, C.-Y.; Lee, C.-Y.; Huang, F.-Y.; Huang, L.-M.J.J.o.t.F.M.A. Influenza pandemics: past, present and future. Journal of the Formosan Medical Association 2006, 105, 1-6, doi.org/10.1016/S0929-6646(09)60102-9.
- Neumann, G.; Chen, H.; Gao, G.F.; Shu, Y.; Kawaoka, Y.J.C.r. H5N1 influenza viruses: outbreaks and biological properties. Cell research 2010, 20, 51-61, doi.org/10.1038/cr.2009.124.
- Cheung, T.K.; Poon, L.L.J.A.o.t.N.Y.A.o.S. Biology of influenza a virus. Annals of the New York Academy of Sciences 2007, 1102, 1-25, doi.org/10.1196/annals.1408.001.
- Zhong, N.; Zheng, B.; Li, Y.; Poon, L.; Xie, Z.; Chan, K.; Li, P.; Tan, S.; Chang, Q.; Xie, J.J.T.L. Epidemiology and cause of severe acute respiratory syndrome (SARS) in Guangdong, People's Republic of China, in February, 2003. The LANCET 2003, 362, 1353-1358, doi.org/10.1016/S0140-6736(03)14630-2.
- Abdel-Moneim, A.S.J.A.o.v. Middle East respiratory syndrome coronavirus (MERS-CoV): evidence and speculations. Springer 2014, 159, 1575-1584, doi:10.1007/s00705-014-1995-5.
- Muyembe-Tamfum, J.-J.; Mulangu, S.; Masumu, J.; Kayembe, J.; Kemp, A.; Paweska, J.T.J.O.J.o.V.R. Ebola virus outbreaks in Africa: past and present. Onderstepoort Journal of Veterinary Research 2012, 79, 06-13.
- Safari, S.; Baratloo, A.; Rouhipour, A.; Ghelichkhani, P.; Yousefifard, M.J.E. Ebola hemorrhagic fever as a public health emergency of international concern; a review article. Archives of Academic Emergency Medicine 2015, 3, 3.
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Gong, F.; Han, Y.; Qiu, Y.; Wang, J.; Liu, Y.; Wei, Y., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 2020, 395, 507-513, doi:10.1016/s0140-6736(20)30211-7.
- Wang, W.; Tang, J.; Wei, F. Updated understanding of the outbreak of 2019 novel coronavirus (2019-nCoV) in Wuhan, China. Journal of Medical Virology 2020, 92, 441-447, doi:10.1002/jmv.25689.
- Nicola, M.; Alsafi, Z.; Sohrabi, C.; Kerwan, A.; Al-Jabir, A.; Iosifidis, C.; Agha, M.; Agha, R. The socio-economic implications of the coronavirus pandemic (COVID-19): A review. International journal of surgery (London, England) 2020, 78, 185-193, doi:10.1016/j.ijsu.2020.04.018.
- Huang, C.L.; Wang, Y.M.; Li, X.W.; Ren, L.L.; Zhao, J.P.; Hu, Y.; Zhang, L.; Fan, G.H.; Xu, J.Y.; Gu, X.Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497-506, doi:10.1016/s0140-6736(20)30183-5.
- Wang, Z.; Yang, B.; Li, Q.; Wen, L.; Zhang, R. Clinical Features of 69 Cases with Coronavirus Disease 2019 in Wuhan, China. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 2020, 10.1093/cid/ciaa272, doi:10.1093/cid/ciaa272.
- Phua, J.; Weng, L.; Ling, L.; Egi, M.; Lim, C.-M.; Divatia, J.V.; Shrestha, B.R.; Arabi, Y.M.; Ng, J.; Gomersall, C.D., et al. Intensive care management of coronavirus disease 2019 (COVID-19): challenges and recommendations. Lancet Respiratory Medicine 2020, 8, 506-517, doi:10.1016/s2213-2600(20)30161-2.
- Prompetchara, E.; Kettoy, C.; Palaga, T. Immune responses in COVID-19 and potential vaccines: Lessons learned from SARS and MERS epidemic. Asian Pac. J. Allergy Immunol. 2020, 38, 1-9, doi:10.12932/ap-200220-0772.
- West, S.A.; Griffin, A.S.; Gardner, A.; Diggle, S.P. Social evolution theory for microorganisms. Nature Reviews Microbiology 2006, 4, 597-607, doi:10.1038/nrmicro1461.
- Huang, H.Y.; Fan, C.H.; Li, M.; Nie, H.L.; Wang, F.B.; Wang, H.; Wang, R.; Xia, J.B.; Zheng, X.; Zuo, X.L., et al. COVID-19: A Call for Physical Scientists and Engineers. ACS Nano 2020, 14, 3747-3754, doi:10.1021/acsnano.0c02618.
- Haldar, J.; Weight, A.K.; Klibanov, A.M.J.N.P. Preparation, application and testing of permanent antibacterial and antiviral coatings. Nature protocols 2007, 2, 2412, doi.org/10.1038/nprot.2007.353.
- Fabbri, P.; Messori, M. Surface Modification of Polymers: Chemical, Physical, and Biological Routes. Modification of Polymer Properties 2017, 10.1016/b978-0-323-44353-1.00005-1, 109-130, doi:10.1016/b978-0-323-44353-1.00005-1.
- Ahirwar, H.; Zhou, Y.; Mahapatra, C.; Ramakrishna, S.; Kumar, P.; Nanda, H.S. Materials for Orthopedic Bioimplants: Modulating Degradation and Surface Modification Using Integrated Nanomaterials. Coatings 2020, 10, doi:10.3390/coatings10030264.
- Nanda, H.S. Surface modification of promising cerium oxide nanoparticles for nanomedicine applications. Rsc Advances 2016, 6, 111889-111894, doi:10.1039/c6ra23046f.
- Nanda, H.S. Preparation and Biocompatible Surface Modification of Redox Altered Cerium Oxide Nanoparticle Promising for Nanobiology and Medicine. Bioengineering (Basel, Switzerland) 2016, 3, doi:10.3390/bioengineering3040028.
- Muñoz-Bonilla, A.; Fernández-García, M. Polymeric materials with antimicrobial activity. Progress in Polymer Science 2012, 37, 281-339, doi.org/10.1016/j.progpolymsci.2011.08.005.
- Poggio, C.; Colombo, M.; Arciola, C.R.; Greggi, T.; Scribante, A.; Dagna, A.J.M. Copper-Alloy Surfaces and Cleaning Regimens against the Spread of SARS-CoV-2 in Dentistry and Orthopedics. From Fomites to Anti-Infective Nanocoatings. Materials 2020, 13, 3244, doi:https://doi.org/10.3390/ma13153244.
- Chu, D.K.; Akl, E.A.; Duda, S.; Solo, K.; Yaacoub, S.; Schunemann, H.J.; authors, C.-S.U.R.G.E.s. Physical distancing, face masks, and eye protection to prevent person-to-person transmission of SARS-CoV-2 and COVID-19: a systematic review and meta-analysis. Lancet (London, England) 2020, 10.1016/s0140-6736(20)31142-9, doi:10.1016/s0140-6736(20)31142-9.
- Odeberg, J.; Wirsén, A.; Norberg, Å.; Frie, J.; Printz, G.; Lagercrantz, H.; Gudmundsson, G.H.; Agerberth, B.; Jonsson, B. A novel cysteine-linked antibacterial surface coating significantly inhibits bacterial colonization of nasal silicone prongs in a phase one pre-clinical trial. Materials Science and Engineering: C 2018, 93, 782-789, doi.org/10.1016/j.msec.2018.08.040.
- Watanabe, Y.; Shuto, Y.; Itoda, Y. Antibacterial/antiviral coating material, and method for forming antibacterial/antiviral coating film. U.S. Patent 9,943,081, 17 April 2018.
- Wen, S.H. Antiviral and antibacterial respirator mask. U.S. Patent 6,681,765, Jan. 27, 2004
- Morse, S.S.; Garwin, R.L.; Olsiewski, P.J. Public health - Next flu pandemic: What to do until the vaccine arrives? Science 2006, 314, 929-929, doi:10.1126/science.1135823.
- Borkow, G.; Zhou, S.S.; Page, T.; Gabbay, J. A Novel Anti-Influenza Copper Oxide Containing Respiratory Face Mask. Plos One 2010, 5, doi:10.1371/journal.pone.0011295.
- Tong, H.W.; Kwok, S.K.C.; Kwok, H.C. Protective masks with coating comprising different electrospun fibers interweaved with each other, formulations forming the same, and method of producing thereof. U.S. Patent 10,201,198, Feb . 12 , 2019.
- Tebyetekerwa, M.; Xu, Z.; Yang, S.; Ramakrishna, S.J.A.F.M. Electrospun Nanofibers-Based Face Masks. SpringerLink 2020, doi.org/10.1007/s42765-020-00049-5.
- Rossett, H. Fluid compositions that can form a coating having antiviral properties. U.S. Patent 13/899,902, 5 June 2014.
- Fox, P.S.; Pedersen, D.E.; Rolando, J.J.; Staub, R.K. Compositions having a high antiviral efficacy. U.S. Patent 8,034,844, Oct. 11, 2011
- Torkelson, A.A.; da Silva, A.K.; Love, D.C.; Kim, J.Y.; Alper, J.P.; Coox, B.; Dahm, J.; Kozodoy, P.; Maboudian, R.; Nelson, K.L. Investigation of quaternary ammonium silane-coated sand filter for the removal of bacteria and viruses from drinking water. Journal of Applied Microbiology 2012, 113, 1196-1207, doi:10.1111/j.1365-2672.2012.05411.x.
