Bone metastasis has been considered the fatal phase of cancers, which remains incurable and to be a challenge due to the non-availability of the ideal treatment strategy. Unlike bone cancer, bone metastasis involves the spreading of the tumor cells to the bones from different origins. Bone metastasis generally originates from breast and prostate cancers. The possibility of bone metastasis is highly attributable to its physiological milieu susceptible to tumor growth. The treatment of bone-related diseases has multiple complications, including bone breakage, reduced quality of life, spinal cord or nerve compression, and pain. However, anticancer active agents have failed to maintain desired therapeutic concentrations at the target site; hence, uptake of the drug takes place at a non-target site responsible for the toxicity at the cellular level. Interestingly, lipid-based drug delivery systems have become the center of interest for researchers, thanks to their biocompatible and bio-mimetic nature. These systems possess a great potential to improve precise bone targeting without affecting healthy tissues.
1. Introduction
Cancer is a major public health issue that reduces the average survival rate of sufferers by 5 years
[1]. It can be of various categories including bone, skin, brain, liver cancer. Among them, bone cancer has been considered one of the most prone sites to metastasis after lung and liver due to its physiological supportive environment for tumor development
[2][3]. Bone metastasis associated with prostate cancers occurs largely in Europe and America comes second after lung cancer in terms of mortality
[4][5].
The bone metastasis takes place in the lumbar vertebrae, thoracic vertebrae, cervical vertebrae, and sacrum parts of the bone, which is not often curable
[6]. Bone metastasis occurs when cancer cells distribute themselves from the site of formation to distantly localized skeletal tissues, and hence, speed up the tumor process. Moreover, red bone marrow allows the entry of cancer cells owing to the anatomy of the skeletal system, where they induce innumerable angiogenic and bone resorption factors
[7][8]. As a whole, the tumor in bones is the result of bone crosstalk by means of chemokines, and some other soluble proteins
[9]. Therefore, unlike bone cancer, which originates from the bones, bone metastasis occurs via cells that break away from different origins. Thus, bone metastasis takes place via hematogenous dispersal
[10]. The dispersed tumor cells facilitate the bone remodeling for the better growth of the tumor cells in the bone marrow. Further, these cancer cells not only initiate osteoclast-mediated bone resorption but also the production of growth factors and the secretion of osteolytic cytokines. This whole event ultimately leads to the formation of osteoblastic lesions in patients with prostate cancers
[6].
Concerning the pathophysiology of bone metastasis, the mechanism of production of osteoblasts (build the bone) and osteoclasts (eat up the bone) is well controlled in a balanced way, known as bone homeostasis. The disproportion in both may result in the shifting of the normal niches into metastatic niches
[11]. Metastatic cancer cells in the bone interact with osteoclasts, which play significant roles in early bone colonization
[12]. The application of anti-cancer drugs, bisphosphonates, radiopharmaceuticals, etc., can be used to cure bone metastasis. However, the non-availability of the ideal treatment strategy is a major concern due to multiple obstacles including bone breakage, reduced quality of life, spinal cord or nerve compression, and pain
[13][14].
Nanomedicine is used as both a diagnostic tool and as a drug carrier to the specifically targeted sites. A variety of benefits of nanotechnology can be derived from treating chronic human diseases by delivering the active molecules to specific sites of action. In this regard, plenty of nano-scale transporters are exploited as nanorobots, nanosensors, and actuation materials in living cells
[15]. Among them, lipid-based nanomaterials have gained great attention worldwide in the research community due to having certain advantages including high biocompatibility, the ability to entrap both hydrophilic as well as lipophilic active molecules, controlled release, as a non-toxic degradation product, to enhance the stability of entrapped molecules, etc.
[16][17]. Moreover, they provide a possibility to design them with surface decorating ligands and different charge-inducing agents
[18]. The lipid nanoformulations meant for drug delivery in cancers have attained remarkable clinical and commercial success (Doxil
®, a liposome formulation encapsulating DOX) and many are still under the clinical trial phase
[19][20][21].
2. Strategies in Bone Targeting
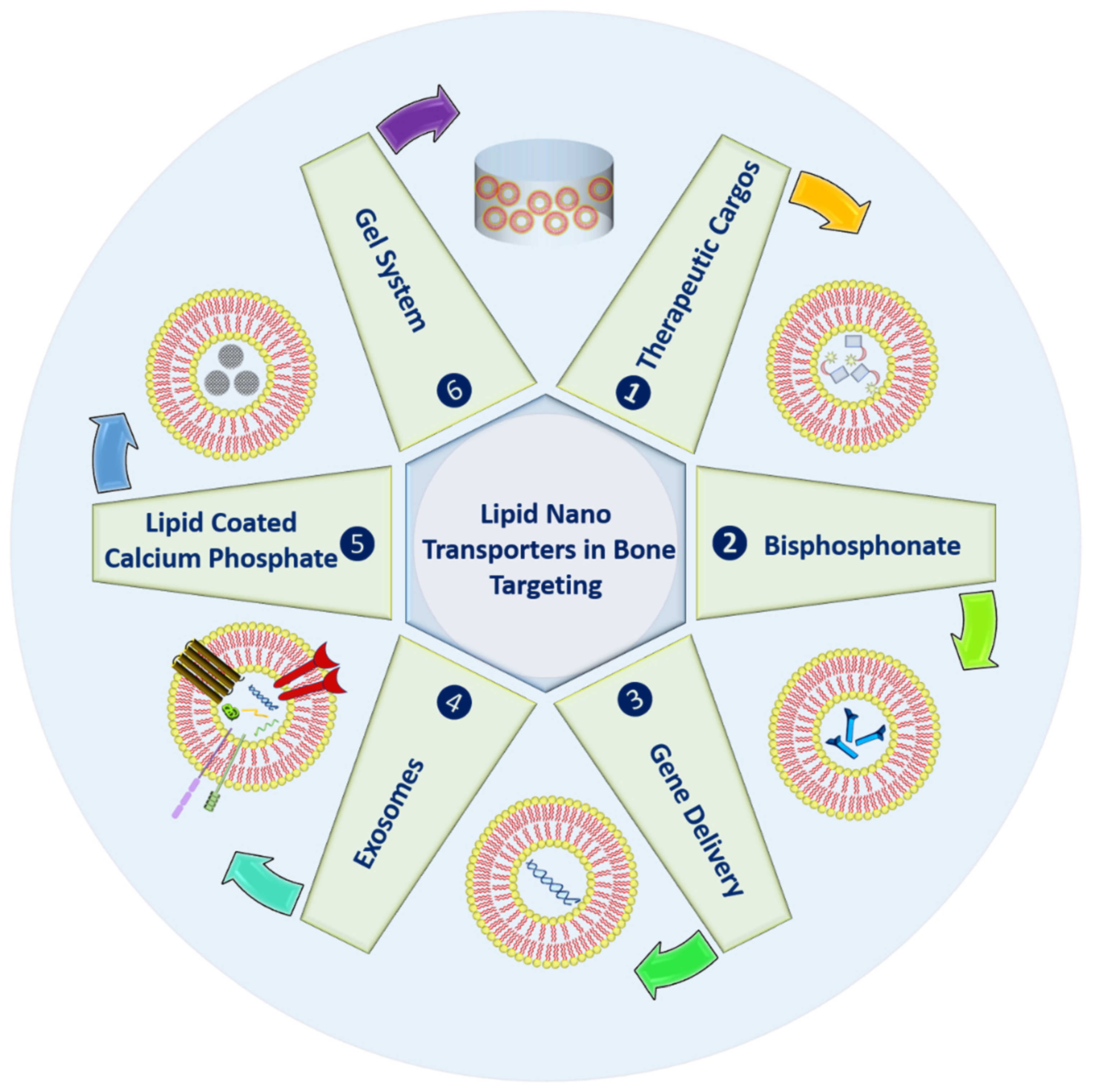
In order to enhance the life of bones, various strategies using lipid-based nanocarriers have been highlighted in Figure 1. These systems can be used to deliver active molecules to the bones for different purposes; they can undergo bisphosphonate conjugation to enhance bone affinity.
Figure 1. Types of the lipid nano-transporters in bone targeting.
Further, lipid carriers can also provide successful gene knockdown due to well know transfection properties. Naturally derived exosomes or extracellular vesicles can be used for various objectives with enhanced shelf life and prolonged circulation. Inorganic materials, namely calcium phosphate, can also be embedded within lipid carriers for bone remolding with improved bone affinity. Finally, to enhance the residence at the bone site, lipid carriers can be gellified. The above-highlighted possibilities have been discussed in detail in the following sections.
2.1. Therapeutic Cargoes
It is very difficult to design a drug delivery system for bone targeting due to the complexity of the solid tumor and the presence of various biological barricades. Some other contributing factors such as hypoxia, pH value of the cell, high interstitial-fluid pressure, physicochemical characteristics of the active drugs, for example, poor water solubility and pharmacokinetic profile, and instability in the harsh physiological environment
[22][23][24].
Furthermore, the physical and chemical limitations of the drug molecules that create hindrance in bone targeting can be solved by applying nanotechnology. For example, liposomes can solve the issues of low water solubility and bioavailability, and avoid first-pass metabolism. Similarly, lipid nanoparticles can enhance bone penetration of the drugs, and prevent the drug from rapid oxidation. Apart from this, zero-order release can be achieved by minimizing the dose frequency. Some of these issues have been addressed and solved, limitations of active molecules and their solutions have been given in Table 1.
Table 1. Issues related to drugs in bone targeting.
| Drug |
Issue |
Formulation |
Outcome |
Ref. |
| Metvan |
Rapid oxidation, interference with blood components |
Nanostructured Lipid Carriers |
Quantitative encapsulation efficiency, sustained-release within 48 h, high cytotoxic effects |
[25] |
| Icariin |
Low water-solubility, susceptible to
first-pass metabolism, and low bioavailability |
Liposomes |
Amplified the mechanical strength of femoral midshaft, triggered bone turnover/remodeling |
[26] |
| Simvastatin |
Deterioration at a physiological pH, low water solubility, low bioavailability, high toxicity |
Lipid nanoparticles |
Higher encapsulation efficiency with a sustained release of 70% within 50 h, reduction in cytotoxicity |
[27] |
| Doxycycline |
Degradation in the anhydrous environment, poor bone penetration |
Lipid- Polymer hybrid system |
Zero-order release rate up to one month, eradicate bacterial bone infections |
[28] |
| Edelfosine |
Poor oral bioavailability, dose-dependent hemolysis |
Lipid nanoparticles |
Shows immediate cytotoxicity to human osteosarcoma cells, negligible tumor growth with declining of tumor volume by five-fold |
[29] |
| TNF-α small interfering RNA |
Short half-life, deprived extravasation from blood vessels to target cells, low cellular uptake |
PEGylated solid-lipid nanoparticles |
Encapsulation efficiency more than 90%, precise targeting to inflamed sites in a mouse model, declined bone loss, |
[30] |
The foremost hindrance in designing a drug transporter is the burst release of the payload before reaching the targeted site
[31]. Undoubtedly, these lipid-based delivery systems have many advantages; still, the biggest challenge is the successful loading of drugs without wasting any fraction and keeping them intact
[32]. The right selection of the type of drug carrier with the best fit composition can keep the entrapped drug molecule stable for more than 6 months, which otherwise may deteriorate in 30 days when available in solution form
[33].
2.2. Bisphosphonate Delivery
Bisphosphonates are non-hydrolyzable pyrophosphate analogous known to interfere with growth and cell signaling by inhibiting the mevalonate pathway
[34]. Alendronate sodium (second-generation bisphosphonate) has been categorized under nitrogen-containing bisphosphonate. It has a very high affinity toward bones, which can reduce bone turnover. Therefore, it can be used as a targeting moiety in bone metastatic treatment strategies
[35].
The presence of bisphosphonates, namely alendronate and zoledronic acid in the drug delivery system can be beneficial in order to attain an improved pharmacokinetic profile of anticancer molecules. These systems can also exhibit sustained high plasma concentration along with slow clearance. In this way, their passive accumulation will ultimately provide a large retention time within the solid tumors
[36][37].
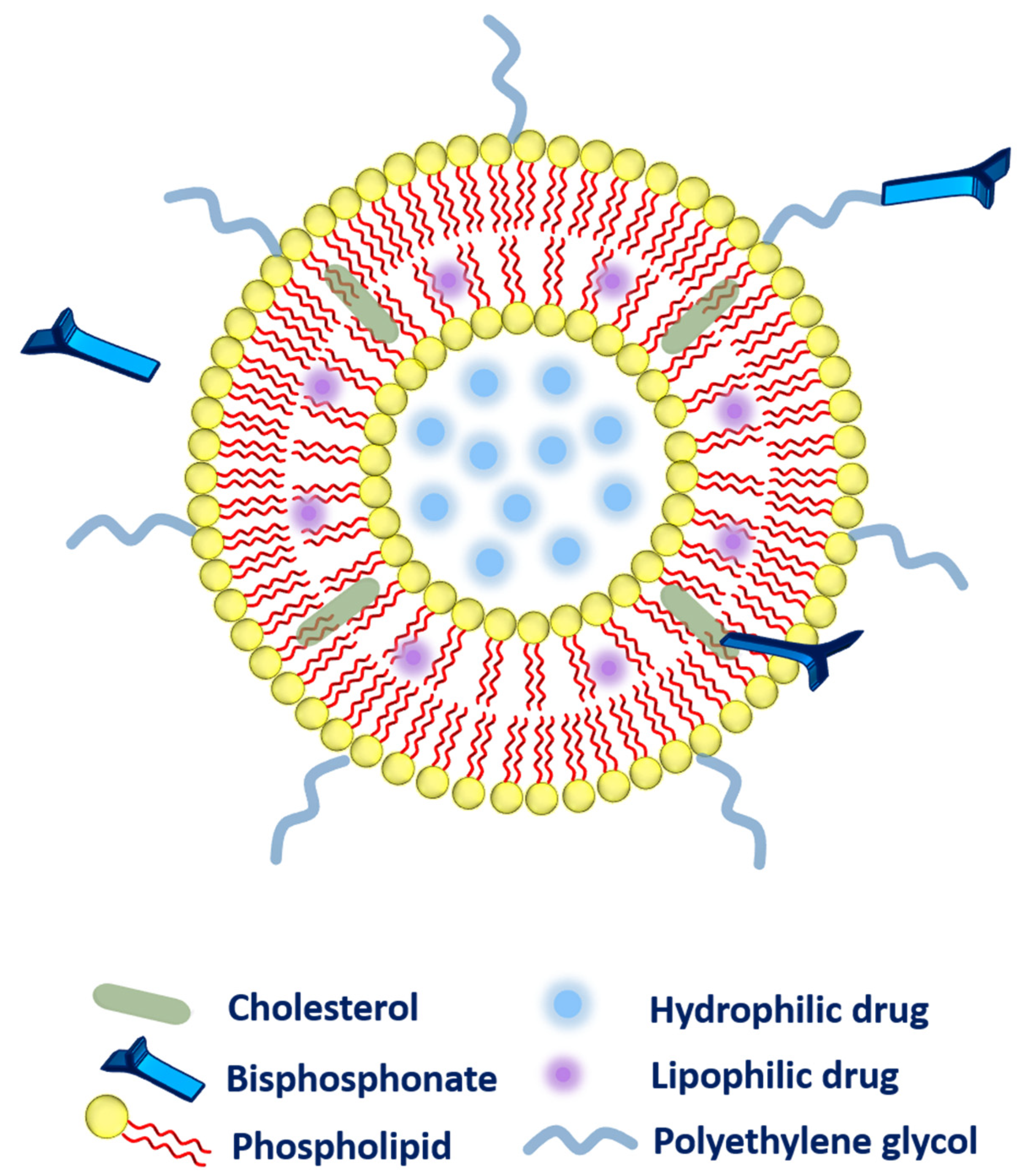
The method of choice to attach the different bisphosphonates to the liposomes or other lipid-based carriers has been conflicting. Several methodologies that have been adopted to link the bisphosphonates to the liposomal system has been highlighted as follow (Figure 2).
Figure 2. Different possibilities for the association of bisphosphonates with the liposomes.
- ➢
-
Firstly, during liposomal production, bisphosphonate (alendronate sodium trihydrate, or zoledronic acid) can be added passively to the aqueous phase of the liposomes owing to high water-solubility
[37][38][39].
- ➢
-
Secondly, the bisphosphonate can also be conjugated along with the cholesterol (as a main component of liposomal composition via a click reaction known as Cu(I)-catalyzed Huisgen 1,3-dipolar cycloaddition). The aforementioned system has exhibited a very strong affinity toward bones
[40].
- ➢
-
Thirdly, bisphosphonate can be associated with the polyethylene glycol (PEG) chain to provide higher circulation time to the liposomes. More precisely, phospholipid-PEG-bisphosphonate conjugation can be employed in liposomes
[41]. Polyethylene-glycol-conjugated phospholipid was used to embed zoledronic acid (as a potent inhibitor of farnesyl-pyrophosphate synthase). The fabricated liposomes were subjected to biodistribution studies that evidenced higher drug accumulation in the liver, spleen, bones, and tumor as compared to zoledronic acid in free form or entrapped in non-PEGylated liposomes. However, toxicity was the main concern as these liposomes were found very toxic to rodents
[36].
Moving ahead, the choice of suitable bisphosphonate is another challenge. Researchers have tried to establish a comparison between different bisphosphonates where liposomal systems composing alendronate have been investigated in T-cell immunotherapy, which delayed ovarian tumor growth in mice. Wherein, a better synergism was established in alendronate and γδ T cells as compared to zoledronic acid. In addition, concerning safety, alendronate has been found safer than zoledronic acid as concluded from cell viability studies. In this manner, the Nitrogen-containing bisphosphonates conjugation has improved the pharmacokinetic profile and enhanced passive retention at tumor sites
[38].
Apart from the liposomes, the solid lipid nanoparticles are also flexible enough to deliver bisphosphonates. One study reported wherein, bisphosphonate has been modified along with the surfactant of choice, which facilitated the transportation of the active molecule outstandingly. It is a well-proven fact that the surfactant is the most crucial component of lipid nanoparticles. Interestingly, Brij 78 (a non-ionic surfactant) has been conjugated with pamidronate (bisphosphonate) in order to enhance the overall bone affinity of the lipid-nanoparticles
[42].
2.3. Gene Delivery
Undoubtedly, bone repair is a natural process. Nevertheless, in most cases, it is not always possible due to the inability of the body itself. In this regard, bone morphogenetic proteins (BMP) play a key role in bone regeneration via regulating cartilage and bone differentiation
[43][44][45][46]. Therefore, the exogenous delivery of these types of growth factors can speed up the repairing process. Nonetheless, there are so many limitations to the delivery of BMP and liposomes can be a good candidate to encapsulate BMP either by their direct addition or transfection through gene carrying. Until now, no study has been found where these two methods have been compared with each other
[47]. Protein delivery faces shortcomings as compared to gene delivery due to major obstacles including high dose, correct folding, and glycosylation of the protein
[48].
In this regard, gene therapy is an approach having the capacity to produce intra-cellular proteins and can express them for a longer period along with the regulation of transgene expression. Moreover, gene therapy avoids the use of a higher concentration of therapeutic moieties; hence, can be delivered only one time with a minimal dose. Henceforth, adverse effects associated with a high amount of therapeutics can be avoided. Therefore, combinational therapy of different genes in association with biomaterials could be the most favorable strategy
[48][49].
Comparing viral gene therapy to non-viral gene therapy is relatively toxic with unpredictable immune responses to the host. Considerably, liposomes with calcium phosphate as a non-viral siRNA vector in gene transfection have been exploited, in which dual functioning is designed firstly to avoid prompt growing of the calcium phosphate particles, secondly to prevent degradation of the entrapped siRNA of the Bcl-2 gene. This proposed system has been designed to deliver the Bcl-2 gene. To make the system pH-triggered charge-reversible, citraconic as a derivative of a maleic amide has been conjugated with cholesterol-aminocaproic acid to coat calcium phosphate particles and the further surface has been tuned by depositing the siRNA. In the last, the positively charged lipid 1,2-dioleoyl-3-trimethylammonium-propanechloridesalt (DOTAP) and negatively charged lipid dioleoylphosphatydicacid (DOPA) has been added and the charge reversal was investigated with the reversal of zeta potential results, which successfully suppress the Bcl-2 without inducing any toxicity in lung cancer A549 cells
[50]. In some cases, the carrier should be negatively charged to achieve efficient transfection whilst positively charged in an acidic endolysosome environment to inhibit its uptake by disrupting the endolysosomal membrane. To tackle this difficulty, the charge reversal phenomenon has been applied
[51][52].
Apart from this, the conjugation between PEG-alendronate accomplished in the stem cells approach has been considered an effective tool in the treatment of various disorders related to bone degeneration due to its osteogenic potential
[53][54]. In this regard, poor bone marrow homing at damaged tissues in response to injury is the main obstacle in the systemic infusion of mesenchymal stem cells (MSC), which have been addressed in a study, wherein the peptide sequence with a strong affinity to bone marrow-derived MSC has been encapsulated into liposomal nanoparticles modified with alendronate sodium (high bone mineral affinity). The aforementioned liposomes were composed of a DSPE-PEG-Alendronate complex for better bone targeting (gene delivery to osteoblastic cells). Further, the biophotonic imaging study assured the successful accumulation of genes in osseous tissue
[41].
2.4. Exosomes
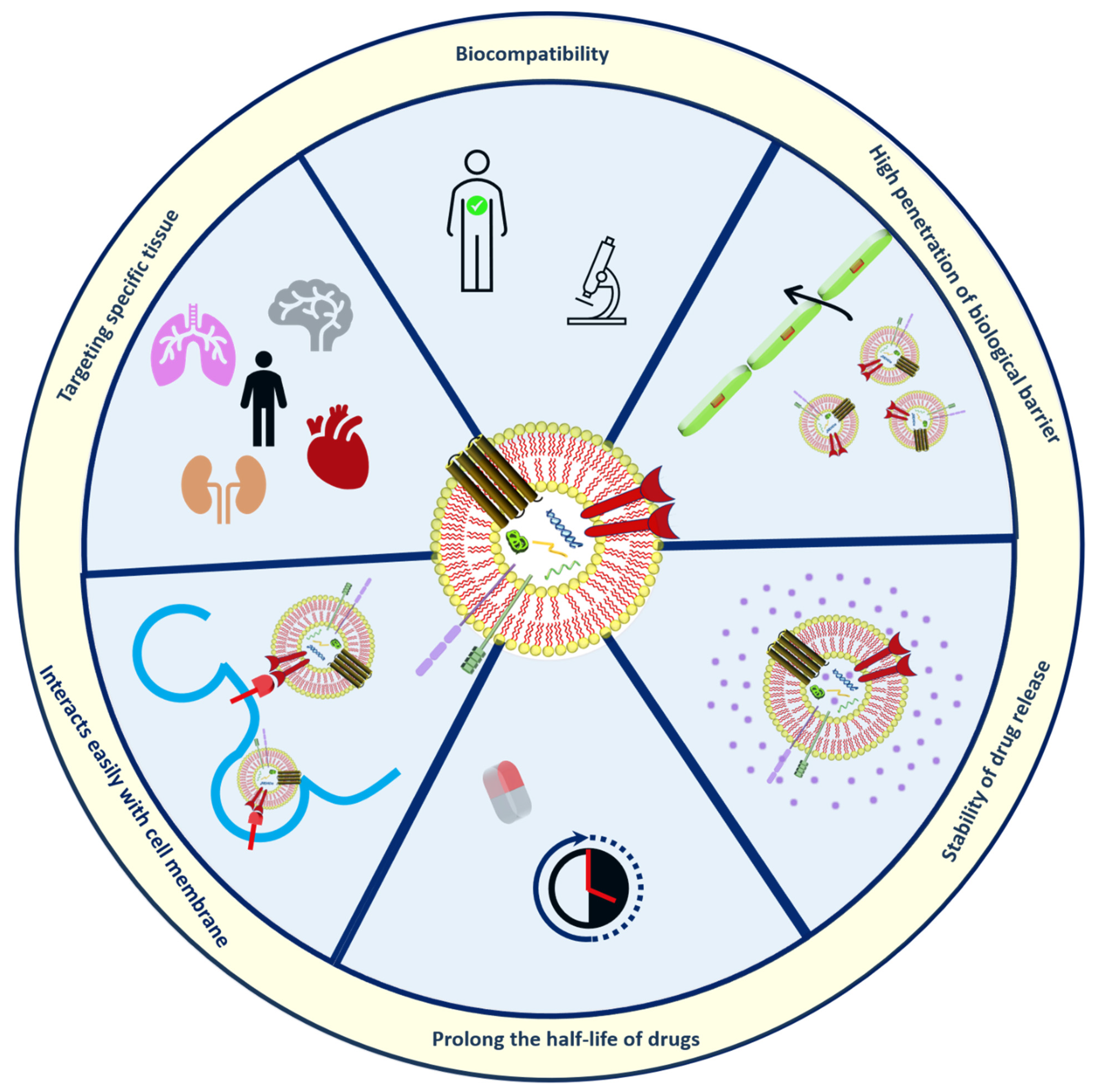
Exosomes (30–150 nm in diameter) as bio-inspired lipid-based novel systems innately originated from biological sources such as endosomal compartments of animal and plant cells utilized as natural transporters between cells by encapsulating nucleic acids, anti-cancer agents, proteins, genetic lipids
[55][56]. The use of exosomes has advantages over artificially prepared nanoparticles discussed in
Figure 3.
Figure 3. Structural organization and Merits of exosomes.
Exosomes are more reliable because of their ability to communicate in the cells naturally
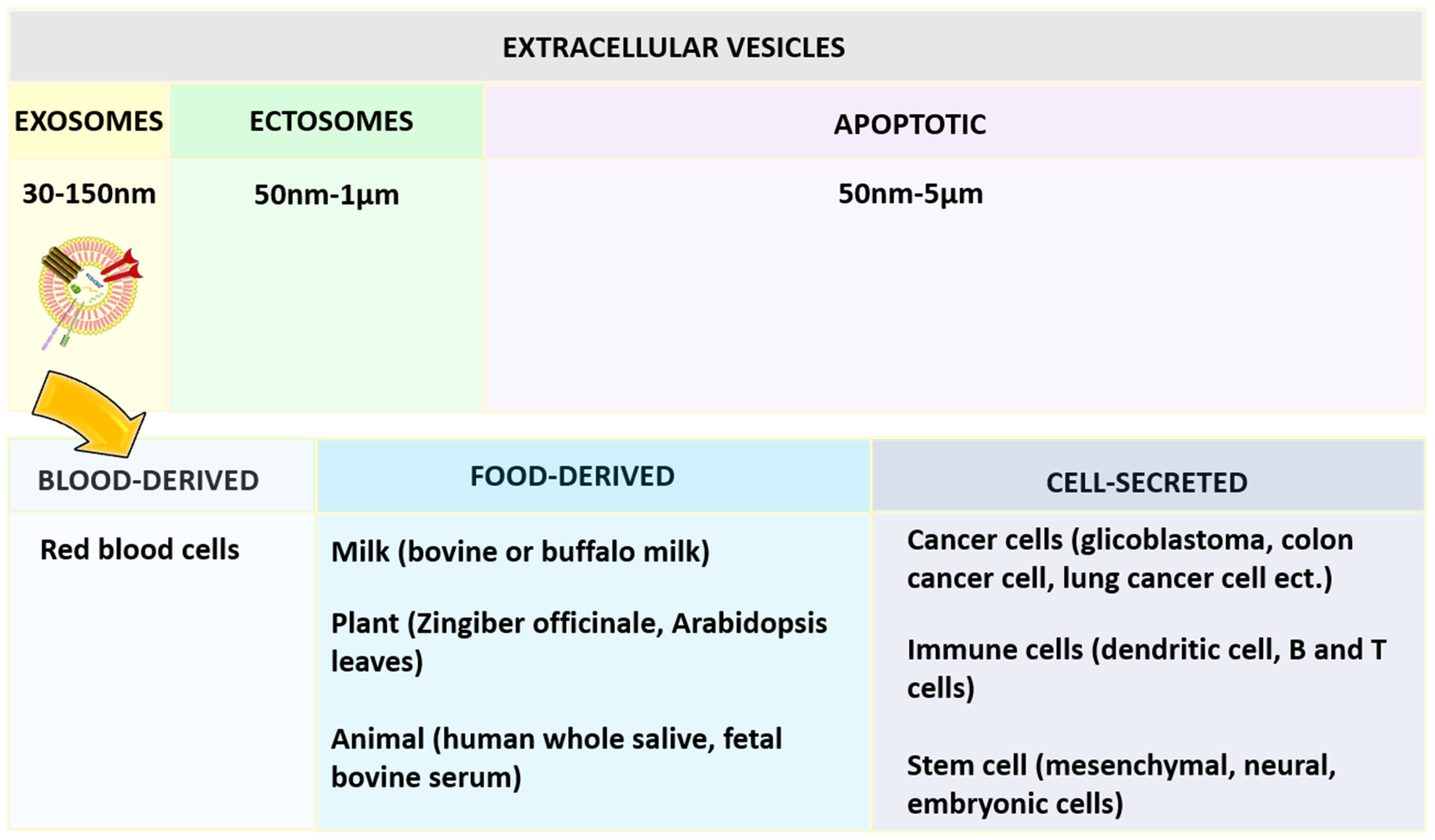
[57]. Based on the diameter, extracellular vesicles can be categorized as shown in
Figure 4, namely exosomes, ectosomes, and apoptotic bodies. Among the exosomes, ectosomes, and apoptotic bodies, the exosomes that are most stable can retain the content enclosed for a longer period and are also useful to enhance the bioavailability of active compounds. They can reach desired sites of action by avoiding various digestive or bio-fluid enzymes. One report has concluded that curcumin was four times more stable by loading into exosomes derived from EL-4 cells
[58][59].
Figure 4. Classification of extracellular vesical based on the diameter and exosomes based on the source of origin.
Exosomes are surrounded by the single external lamela, originating largely from blood, food, and the cell (
Figure 4) and widely distributed in plasma, urine, semen, saliva, bronchial fluid, breast milk, serum, amniotic fluid, synovial fluid, tears, lymph, bile, and gastric acid
[60]. In the beginning, exosomes were considered waste material from cell damage and cell homeostasis, later they were proposed to carry therapeutic moieties
[61].
Fascinatingly, the exosomes can disclose the biological information of the eukaryotic cells from where they have been collected practically, which is useful in curing a wide range of diseases such as chronic inflammation, specifically tumors
[62][63].
Exosomes are developed from the inward folding of the membrane of the early endosomes (originating from the plasma membrane of the cells), later giving rise to multivesicular bodies and taking part in various endocytic and trafficking tasks
[60][64]. Further, the high cholesterol fraction containing multivesicular bodies will be guided toward fusion along with the plasma membrane to release exosomes that otherwise undergo destruction by lysosomes. Therefore, this whole event depends on the amount of cholesterol content present in multivesicular bodies
[60][65]. Exosomes possess a very complex architecture containing 4400 proteins, 194 lipids, 1639 mRNAs, and 764 miRNAs, which depicts their functional diversity
[66][67].
Attributable to the complexity of the exosomes based on the structural organization (huge number of proteins), no robust strategy is available to provide their reproducibility. Another possible reason is the variation of exosomes derived from different matrixes. Therefore, a set of different techniques need to be applied, which increases the overall production cost. These are the frictions in the smooth production of exosomes that escalate their use at the clinical level. In general, ultracentrifugation is used for all types of exosomes collections while other methods are also available based on size, immunoaffinity capture, and precipitation of exosomes. Still, these methods are not sufficient enough for the purification of exosomal yield because the result is usually a mixture of non-differentiated content of exosomes and different extracellular vesicles
[60].
Moreover, in the future new strategies may be evolved involving immunoaffinity and microdevices along with good outputs and higher purified collection. Stremersch, S and co-authors have compared not only the different methods of loading to exosomes (either during biogenesis or after isolation of exosomes) but also to synthetic liposomes for the fusion capacity. No remarkable difference has been noticed in the uptake of all the above-mentioned systems. This study proposed that anionic liposomes were able to target gene knockdown under the same experimental conditions; however, exosomes cannot because of inappropriate/no release of encapsulated miRNA
[68].
Liposomes and exosomes are closely related in terms of composing one lipid bilayer, a diameter below 200 nm, and the capability to load both hydrophilic and lipophilic active moieties. In the case of discrepancy, exosomes offered a more complex surface with high specificity due to the existence of numerous proteins (such as tetraspanins). In a typical composition of liposomes, no proteins are associated with the surface of the external membrane. These proteins make possible the efficient targeting and uptake hence, have very good immunocompatibility
[69].
Although, exosomes face some disadvantages including rapid clearance, low loading capacity, and the non-availability of scale-up manufacturing techniques. In contrast, liposomes provide the possibility for PEGylation to achieve long circulation, well-defined production even at an industrial scale (many liposomal products are already on market), and the feasibility of the ligand-guided approach
[69][70].
Despite this fact, it has been always a topic of conflict that liposomes are better than exosomes comparatively and vice versa. To neutralize the above statement, the lipid component of liposomes can be used in exosomes to achieve higher intelligence concerning membrane fusion
[71][72]. Therefore, the functioning of liposomes and exosomes merging into one system has been investigated. Herein, the surface membrane protein of exosomes hybridized not only along with the functional lipids of liposomes but also PEGylation did enhance the overall colloidal stability of the system. These two systems can be combined through processes namely freeze–thaw, incubation, and sonication
[73].
Bone-derived exosomes in bone-related complications can play a key role in the regulation of gene expression, migration, and proliferation. In prostate cancer, affected cells secrete both osteoblast and osteoclast stimulating factors that initiate either bone resorption or bone formation, or both. The bone metastasis disturbs the balance between the events of bone-resorbing osteoclasts and bone-forming osteoblasts
[74]. In general, bone metastasis-related to prostate cells is osteoblastic in radiographs and exosomal microRNAs derived from the prostate cancerous cells are believed to promote osteoblastic bone metastasis
[75][76]. Osteolytic lesions have been traced in all the subjects affected with the osteoblastic metastasis
[77]. To understand this mechanism, the role of exosomes derived from osteoblastic, osteoclastic, or combination of human prostate cancer cells have been identified in one study. Wherein, investigators have found that exosomes promoted bone tumor growth via osteoclastogenesis in vitro and induced osteolysis in vivo. MicroRNA delivered through exosomes has inhibited osteoblastogenesis evidenced by inhibition of type I collagen expression in vivo. Hence, aggressive growth of prostate cancer cells inhibited, deteriorated bone matrix, and induced premetastatic niche for tumor growth, thus, playing a key role in bone homeostasis
[75].
Exosomes can also be used in bone regeneration using exosome-loaded scaffolds. The incorporation of the exosomes into the scaffolds is completed via physical adsorption (incubation of scaffolds in exosomes solution)
[40][78] and in situ gelation via cross-linking
[79].
This entry is adapted from the peer-reviewed paper 10.3390/nano12071146