Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
In the last few decades, the vast potential of nanomaterials for biomedical and healthcare applications has been extensively investigated. Several case studies demonstrated that nanomaterials can offer solutions to the current challenges of raw materials in the biomedical and healthcare fields. This review describes the different nanoparticles and nanostructured material synthesis approaches and presents some emerging biomedical, healthcare, and agro-food applications.
- nanomaterials
- risks and toxicities
- emerging applications
1. Introduction
In the last 50 years, material researchers have been extensively studying how to exploit nanoparticles and nanostructured materials in different biomedical and healthcare sectors [1]. The term “NP” usually defines minute particles of matter (1 to 100 nm in diameter), but other names can be used to describe larger particles (up to 500 nm in diameter). For example, nanorods, nanowires, and nanofibers are nanoparticles with a diameter in the 1–100 nm range but with one dimension outside the nanoscale dimension [2]. Nanostructured materials are nanomaterials with one dimension in the nanoscale range (<100 nm) and are made of a single material or multiple materials. Therefore, nanostructured materials are composed of interlinked parts in the nanoscale range [3]. Nanoparticles and nanostructured materials can be made of simple materials (e.g., metal, carbon, polymer) [4], of composites (e.g., polymer-metal, silica-metal, graphene-metal), or in the core-shell form [5][6][7][8]. Nanomaterials are typically synthesized by one of two main approaches, i.e., bottom-up approach and top-down approach. Among all the methods, recently, the synthesis of nanomaterials by physical vapor deposition, chemical vapor deposition, electrospinning, 3D printing, biological synthesis, and supercritical fluid have gained importance, which is mingled with other methods to improve the synthesis efficiency [9][10]. Nanomaterials display many interesting features, such as superior mechanical performance, the possibility of surface functionalization, large surface area, and tunable porosity, compared to their bulk materials [11][12][13]. These outstanding features explain why nanomaterials are the perfect candidates in the biomedical sector for the production of tissue-engineered scaffolds (e.g., blood vessels, bone), drug delivery systems (gene therapy, cancer treatments, drugs for chronic respiratory infections), chemical sensors [4][5], biosensors [6][7], and wound dressings [14][15]. Remarkably, several studies suggest that ancient civilizations in India, Egypt, and China used nanotechnology (metallic gold) for therapeutic purposes in 2500 BC [16]. Nanomaterials’ discrete features can complicate the assessment of the effects and the toxicity risk associated with their use in a biological environment. Indeed, nanomaterials’ chemical composition, size, shape, surface charge, area, and entry route in the body can influence their biological activities and effects [17]. Considering the growing use of nanomaterials over the last decades, our group reviewed the key aspects of different types of nanomaterial design and their emerging applications in the biomedical fields [1][2][3][15]. This review article discusses the unique features of nanomaterials that are exploited for different biomedical applications (Figure 1).
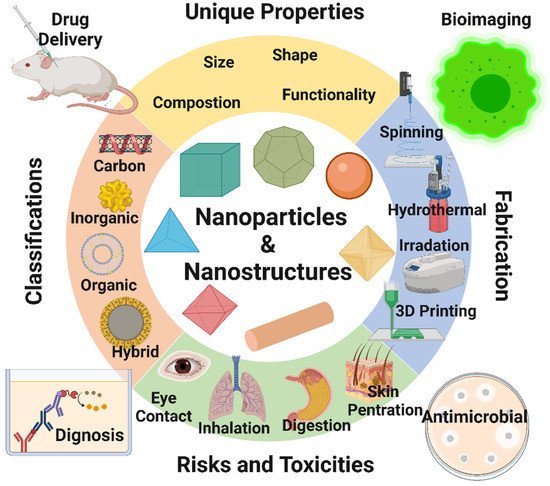
Figure 1. Summary of the recent topic on nanoparticles and nanostructured materials and their applications in bioimaging, biosensing, drug delivery, tissue engineering, antimicrobial, and agro-food sectors. Image created by Biorender.
2. Fluorescent Nanomaterials for Bioimaging
Bioimaging is an advanced non-invasive technology used to visualize internal structures and physiological processes in living cells/organisms in real-time. It is a safe and effective technique for monitoring biological functions without affecting normal life processes (e.g., respiration and movement). It also helps to obtain data on the sample 3D nanostructure [18] and to investigate tissues at the subcellular and multicellular scale [19]. Several nanoprobes have been developed for bioimaging and the treatment of many diseases (cancer, heart, and inflammatory diseases) [20][21][22]. Nanomaterials are ideal materials for nanoprobes because they can be exactly characterized using nuclear magnetic resonance or gel permeation chromatography and are easily secreted from the body. However, their functions are limited, and researchers are always looking for new materials. In recent days, magnetic nanoparticles have gained great interest because of their progress in image-guided therapy (e.g., fluorescence, magnetic resonance, X-ray CT) and cancer theranosis favorable properties, such as tunable size, generating reactive oxygen species (ROS) or heat, simple fabrication, energy transfer, and X-ray absorption properties (Figure 2). Moreover, their long-term toxicity and dispersion stability must be specifically investigated.
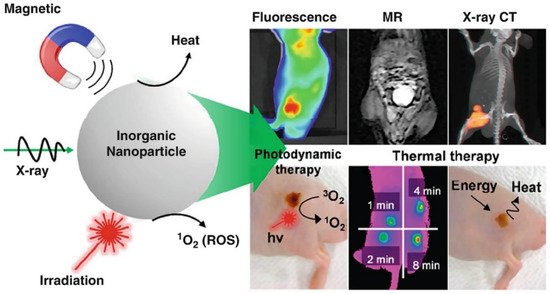
Figure 2. Schematic representation showing the utilization of magnetic nanoparticles in tumor bioimaging and therapy [23]. Copyright 2016, American Chemical Society.
3. Nano-Drug Delivery Systems
Drug delivery systems are quite new, but is a rapidly expanding technology. In these systems, nanoscale materials are used to deliver the therapeutically active drug or the imaging molecule (when used as diagnostic tools) to the targets [24]. Nanostructures (made of metals, organic/inorganic, and polymeric materials) are often used for the development of drug delivery systems (Figure 5). Nanoparticles and nanostructured materials are crucial components in these carrier systems that play a key role in personalized medicine by improving drug formulation/targeting/controlled release [24][25]. Such systems can deliver a drug to a specific site at a predetermined rate and in a predesigned manner; consequently, the drug bioavailability will be enhanced, while side effects will be reduced. Drugs can be physically or chemically adsorbed into the nanoparticles surface through various adsorption methods, or they can be loaded on nanoparticles during their production [24][26]. The drug and carrier properties, such as drug-carrier solubility, molecular weight, drug-carrier chemical interaction, and carrier size, will determine the drug loading efficacy on/into the carrier [24][27]. The drug release rate from the nanoparticlesis mainly influenced by (1) the release of the adsorbed drug from the surface of the nanoparticles; (2) the drug diffusion from thenanoparticles; and (3) the nanoparticle erosion and drug diffusion from the nanoparticles. Therefore, the drug release rate from the nanoparticles will be governed by polymer biodegradation and drug diffusion. The drug release time and location can be modulated by the nanoparticles composition (e.g., thermosensitive and pH-sensitive materials) and engineering (e.g., monolayer and multilayer nanoparticles, nanocapsules), and also by better understanding the physiological factors involved in this process [24][28] (Figure 5).
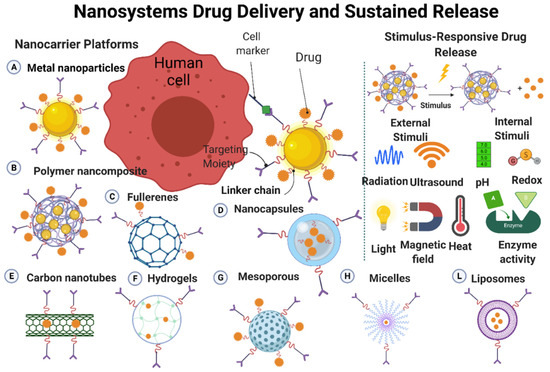
Figure 5. Characteristics of nanomaterials that can cross the biological membranes to deliver a drug to a specific site and mechanisms influencing controlled drug release. Image created by Biorender.
4. Antimicrobial Materials
Antibacterial agents are used in the biomedical sector, textile industry, water treatment, and food industries [29]. The antimicrobial characteristics of nanoparticles are influenced by several factors [30], including size, shape, and the type of encapsulated antibiotics drug. For instance, nanoparticles with angular shapes (e.g., triangular, cubic, tetrahedral) cause mechanical harm to the microbial membrane, and this contributes to increased microbial growth inhibition compared to spherical nanoparticles [31]. Based on their antimicrobial properties, antimicrobial nanoparticles can be classified into four main categories: antibacterial, antifungal, antiviral, and antiparasitic nanoparticles [32]. Surface functionalization allows the production of antibacterial nanoparticles with two different antibiotics to concomitantly kill, for instance, Gram-positive (encapsulated) and Gram-negative (attached) bacterial strains. Inorganic disinfectants, such as metal oxide nanoparticles, are gaining popularity because of the limitations of organic disinfectants, such as toxicity to humans [30][33]. Currently, nanophysics researchers are investigating the effects of different metallic nanoparticles in bacteria. The antibacterial mechanism of metallic nanoparticles is still debated, but three major mechanisms are proposed: (i) reactive oxygen species (ROS) formation; (ii) metal ion release from metallic nanoparticles; and (iii) metallic nanoparticles interaction with the cell membrane. Metallic nanoparticles display higher antibacterial effects than their salts. The antibacterial function is often influenced by the size of the metallic nanoparticles [34]. The mechanisms whereby these factors can modify the antibacterial activity of Ag NPs are many (Figure 7) and include ligand replacement, oxidative dissolution, Ag+ ions reduction, passivation of the Ag surface, passivation layer puncturing, silver speciation, and nanoparticle aggregation [206]. These phenomena may also be influenced by some chemical species. For instance, chloride can accelerate or slow down corrosion in the function of its concentration. Therefore, the antimicrobial activity of Ag NPs should be assessed in controlled conditions to limit/prevent unexpected changes in the system. Specifically, Ag NPs must be stored in the dark and without oxygen. Antibacterial mechanisms can be classified in two groups: non-oxidative and oxidative mechanisms (Figure 7).
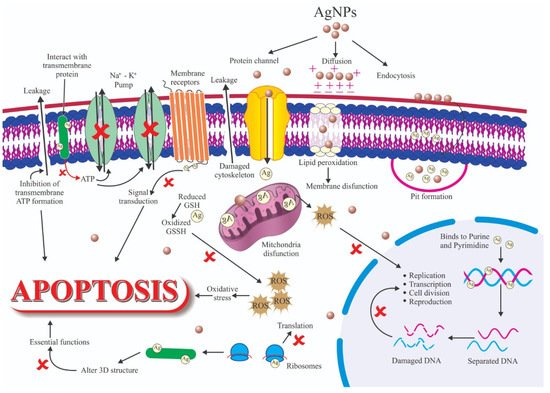
Figure 7. Schematic representation of several factors that influence silver nanoparticles’ (Ag NPs) antibacterial activity.
5. Gene Therapy
Gene therapy is a method in which genes are modified to prevent and/or treat disease. With this revolutionary technique, clinicians can treat a disease simply by incorporating the modified gene into the patient’s cells, without the need for surgery or drugs. Depending on the disorder, gene therapy may be used to add a functional copy of a gene that is not working properly or to switch off the gene that is causing the problem. These modified genes are delivered into the cells using a vector (i.e., a genetically modified transporter). Due to their nanometric size, large surface-to-volume ratio, and stability, nanoparticles are attractive agents as gene carriers [24]. Designing nanoparticles for gene delivery is not easy because many factors must be taken into account. First, nanoparticle functionalization should bring biocompatible layers and contribute to maintaining the structural integrity and activity of the transported genes/drugs in the biological fluids. Second, nanoparticle production, like for any therapeutic product, must take into account the following issues: physicochemical properties, biopharmaceutical properties, and pharmacological properties. Therefore, nanoparticles should have the capacity to treat directly, and this requires new design parameters. Studies on the Administration, Distribution, Metabolism, and Excretion (ADME) of nanoparticles should be designed to take into account their aggregation and surface characteristics (Figure 8) [35].
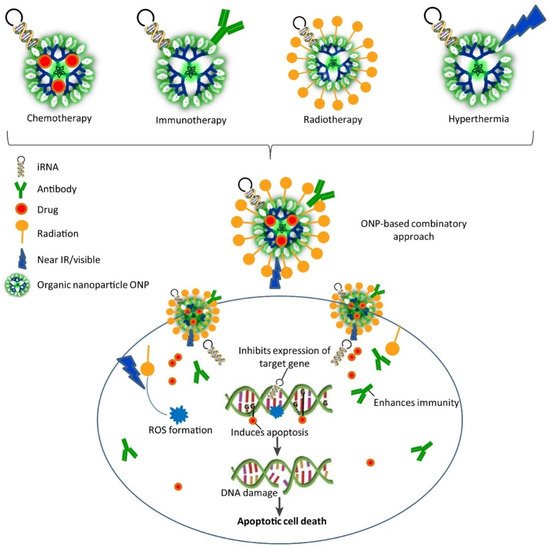
Figure 8. Combinatorial approaches based on organic nanoparticles (ONP) for gene therapy are associated with other therapies [36]. Copyright 2017, Trends in Biotechnology.
6. Biosensors
Biosensors are devices that combine organic components (e.g., antibodies, enzymes) and an electronic element to yield a detectable signal that can be quantified [37]. The electronic elements detect the physiological change produced by the interaction of the organic component with environmental chemical or biological elements [38][39]. A typical biosensor consists of five main components (Figure 9): (i) the analyte, a substance the presence and amount of which are detected [40]; (ii) the receptor, an organic molecule that can detect the analyte [39]; (iii) the transducer, a device that converts the physiological change occurring following the analyte-receptor interaction to a quantitatively measurable optical or electrical signal [38]; (iv) the electronic part that receives and quantifies the signals from the transducer; and (v) the display, an interpretation system (a computer and a printer) to display the response output in a manner that can be understood by the user [41].
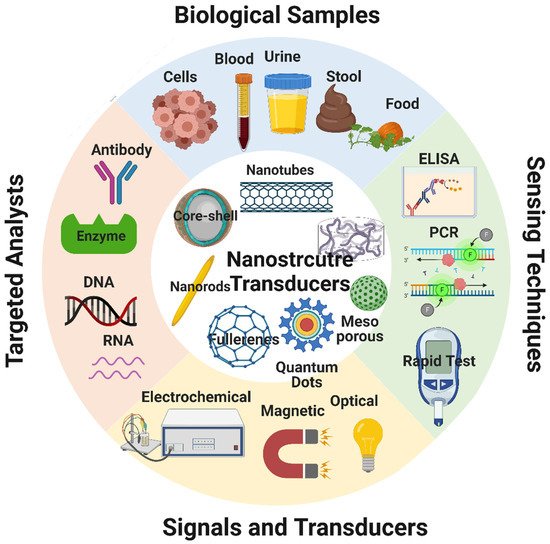
Figure 9. Schematic representation of the components of a typical biosensor and of the different types of bioreceptors and transducers. Image created by Biorender.
7. Tissue Engineering
Tissue engineering is a multidisciplinary approach to develop structures that are made of biological components (e.g., cells, stimulatory molecules) and biomaterials that can mimic the native organ/tissue. Engineered tissues may be used at the place of conventional organ/tissue transplant procedures to lower the cost burden [42][43]. The rapidly evolving nanotechnological and fabrication techniques have allowed the incorporation of various biocompatible nanomaterials in tissue engineering, including nanoporous scaffolds and nanofiber membranes [44][45]. Nanomaterials have been used in various tissue engineering applications (periodontal, neural, bone, and skin tissue engineering) [46]. Nanomaterials can contribute to finely tuning the scaffold characteristics, particularly the mechanical strength, and regulating the release of bioactive molecules (growth factors, cytokines, inhibitors, genes, drugs) [47][48]. Nanofibrous materials are gaining interest for tissue engineering applications, including skin regeneration. Nanofibrous structures mimic the native extracellular matrix and promote the adhesion of various cells and soluble factors that may promote cell function and tissue regeneration (Figure 11) [248].
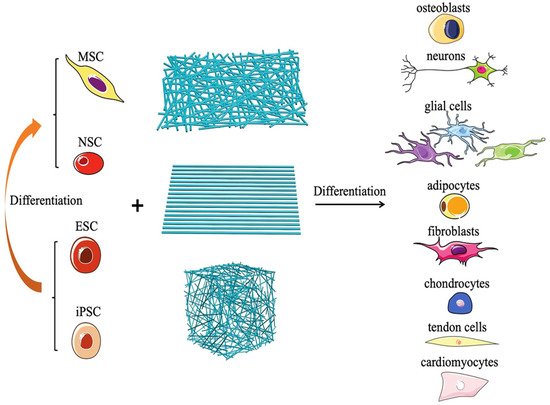

Figure 11. Schematic figure to show how electrospun nanofibers promote the differentiation of various types of pluripotent stem cells into different lineages [49]. Copyright 2020, Wiely.
8. Agriculture and Food Industry
Nanotechnology is used in agriculture to enhance food production and also to improve/preserve the nutritional content, quality, and safety of foods. Fertilizers, insecticides, herbicides, and plant growth factors/regulators are used to enhance agricultural yields. Nanotechnology is also more and more implicated in the development of approaches to stimulate seed germination, plant growth, and plant defenses [50]. Metal nanoparticles (Ag NPs and Cu NPs) have been particularly studied in plant science. Their organic synthesis is very expensive and involves dangerous chemicals [51]; however, nanoparticle surface functionalization allows the accommodation of more micronutrients in one nanoparticle for efficient delivery to the plants. These micronutrients may enhance productivity and increase the nutrient content in agriproducts. Carbon nanomaterials are commonly used in agriculture because they can influence the plant’s metabolic functions, and ultimately, its growth. Therefore, these nanomaterials, used at very small concentrations, can go into the plant cells and could be an effective answer to increase crop yield and fruit production [52]. Figure 12 shows some potential applications of nanomaterials in the animals and agriculture industry, i.e., nano-fertilizers and nano-pesticides, which stimulate animal and plant growth using nanomaterials; smart monitoring for animals and plants using nanosensors by wireless communication devices and smart packaging.
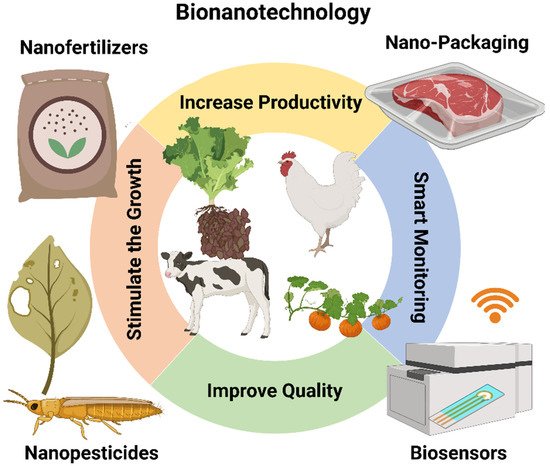
Figure 12. Potential applications of nanomaterials in the animal and agriculture industry. Increase the productivity of the crop using nano-pesticides and smart packaging; Improve the quality of the soil using nano-fertilizers; Stimulate animal and plant growth using nanomaterials; Provide smart monitoring for animals and plants using nanosensors by wireless communication devices. Image created by Biorender.
9. Risks of Exposure to Nanomaterials
Despite their advantages, nanomaterials may also be associated with risk factors. Nanoparticles can adversely affect different organs/tissues in the body and can be associated with different disorders (Figure 13). Nanoparticle exposure can promote the development of neurological disorders (e.g., Parkinson’s disease and Alzheimer’s disease), lung (asthma, bronchitis, emphysema, and cancer), and cardiovascular diseases (atherosclerosis, arrhythmia, thrombosis, and hypertension). In addition, exposure to nanoparticles can cause skin irritation, dermatitis, urticaria, and other skin problems.
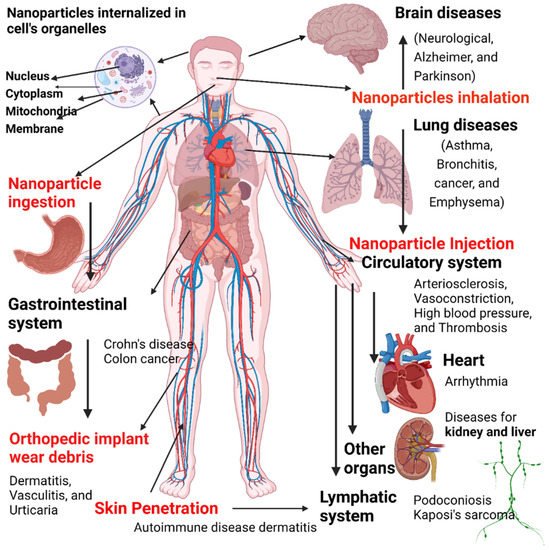
Figure 13. Disease caused by exposure to nanoparticles and entrances of nanoscale materials into the body through inhalation, dermal exposure, and ingestion, resulting in many potential hazards. Image created by Biorender.
10. Conclusions
Nanomaterials are interesting materials because of their superior and tunable physical, chemical, and biological features compared with bulk materials. Nanomaterials can be classified in function of their size, shape, composition, and origin. Researchers have exploited nanomaterial features by grafting different groups on them, thus making nanoparticles suitable for biomedical applications. In this review, we presented the applications of nanomaterials in bioimaging, skincare, tissue-engineered scaffolds, drug delivery systems, biosensors, and wound healing, as well as in the food and agricultural industries. The use of nanomaterials for targeted drug delivery has also dramatically progressed with exceptional applications to reduce the limitations of conventional drug delivery systems. Different nanomaterial types (e.g., spherical nanoparticles, core-shell, nanorods, nanowires, hollow, nanofibers, and mesoporous) are studied for the targeted delivery of drugs. Encapsulation techniques have also been tested to deliver various bioactive cytotoxic agents. Nanoparticles also have their place in tissue reengineering for the repair of various tissues. Thanks to their larger surface-area-to-volume ratio, nanostructured scaffolds can act as selective substrates to absorb specific proteins and promote cell adhesion. Carbon and metal-based nanoparticles for biosensor development can lead to many applications in agriculture and in the biomedical sector. The review also presented the fluorescence nanoparticles and the in vivo and in vitro fate of nanoparticles. The applications of fluorescence nanomaterials in bioimaging were explained clearly. The antimicrobial activities of various nanomaterials and their advantages and disadvantages are also discussed individually
This entry is adapted from the peer-reviewed paper 10.3390/nano12030457
This entry is offline, you can click here to edit this entry!
