Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Neurosciences
Insulin action in the brain regulates several processes including energy expenditure, glucose homeostasis, feeding behavior and satiety, reward pathways, reproduction, cell proliferation and differentiation. Moreover, insulin has neuroprotective and neuromodulatory properties and plays a crucial role in neuronal transmission and survival, neurogenesis, plasticity, and memory and cognition.
- insulin signaling
- metabolic disorders
- neurodegeneration
- cognition
1. Role of Insulin in Brain Glucose Metabolism and Feeding
Glucose is the main source of energy in the brain, reaching this organ through the GLUTs, which have numerous isoforms [6]. Most glucose uptake to brain and by neuronal and glial cells is insulin-independent and relies on the insulin-insensitive GLUT1 and GLUT3, among other less abundant region-specific GLUTs [79,80,81]. However, in brain regions related to cognition and metabolic control, such as the basal forebrain, hippocampus, amygdala, cortex, cerebellum, and hypothalamus, the inducible insulin-sensitive GLUT4 is reported to be co-expressed with GLUT3 [82,83,84]. Activation by insulin induces the translocation of GLUT4 to the membrane and is thought to improve glucose flux into neurons during periods of high metabolic demand, such as learning [85,86]. The effect of insulin and its relevance on glucose uptake in the brain is not consensual and for a long time was thought to be exclusively insulin independent. In fact, several studies from the 80′s that focused on the effect of insulin in brain glucose uptake showed that brain glucose metabolism was unaffected by insulin. Goodner et al. measured glucose uptake in fasting rats 30 min after 0.1U insulin administration [87] and observed that the brain did not increase the rate of glucose uptake, concluding that the brain was “insensitive to insulin” [88]. However, subsequent studies using 18-fluorodeoxyglucose positron emission tomography showed that insulin increases brain glucose uptake in humans, mostly marked in cortical areas [89]. In agreement with the role of insulin in regulating glucose uptake in the brain, GLUT4 colocalizes with IR in several brain regions [90].
Another important role of insulin in the indirect control of glucose homeostasis is related with the mesolimbic reward pathways on the dopaminergic neurons [91]. Insulin can alter food choices and preferences, as it increases dopamine release in the reward pathways after food restriction, an effect not seen in animals submitted to hypercaloric diets. Therefore, it has been shown that dopaminergic pathways are negatively regulated by insulin, as insulin causes the desire to consume high-calorie foods rich in sugar and fat, promoting a feeling of satiety and reward [91,92].
Beyond its role on controlling glucose homeostasis, insulin in the brain also impacts insulin sensitivity at the periphery. Heni and colleagues have found in lean men that central insulin action, achieved by an intra-nasal insulin spray application, improved peripheral insulin sensitivity measured by hyperglycemic clamp [93]. When they explored the mechanisms involved, they discovered that the vagus nerve and the parasympathetic system activation is involved in the peripheral insulin sensitivity regulation by CNS insulin. The hypothalamus appears to be one of the most important brain regions in this link [94]. Moreover, they have found an impairment in the central regulation of peripheral insulin signaling in obese people, showing the tight link between brain and peripheral insulin resistance in dysmetabolism conditions [94].
Apart from glucose homeostasis regulation, insulin and its receptors have implications in the regulation of energy balance where the hypothalamus also plays a major role [18]. Together with insulin, leptin, an adipokine produced by the adipose tissue, plays a role in the regulation of feeding behavior. Both hormones act on the hypothalamus affecting the expression of neuropeptides involved in satiety pathways [18,95]. In the hypothalamus, these hormones act in the arcuate nucleus (ARC) which is composed by two antagonistic types of neurons: the anorexigenic neurons or the appetite-suppressing neurons known as proopiomelanocortin-expressing neurons (POMC) neurons, and the orexigenic or appetite-stimulating neurons known as neuropeptide Y (NPY) and agouti-related peptide (AgRP) expressing neurons [18,95] (Figure 2). Both orexigenic and anorexigenic neurons express insulin and leptin receptors.
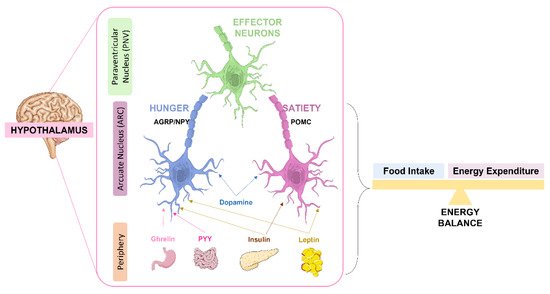
Figure 2. Role of the hypothalamus in the regulation of energy balance. Signals as leptin and insulin act antagonistically in the two antagonistic neurons of the arcuate nucleus (ARC): the orexigenic (appetite-stimulating) neuropeptide Y (NPY) and agouti-related peptide (AgRP)-expressing AgRP/NPY neurons and the anorexigenic (appetite-suppressing) proopiomelanocortin (POMC)-expressing POMC neurons. By one way, insulin and leptin stimulate POMC neurons, in another way they inhibit AgRP/NPY neurons. Both AgRP/NPY neurons and POMC neurons project to second-order neurons in the paraventricular nucleus (PVN), leading to an integrated response on energy intake and expenditure. The intestine also secretes peptide YY (PYY) and the stomach ghrelin that act on AgRP/NPY neurons to stimulate hunger. Dopamine modulates both AgRP/NPY and POMC neurons.
The POMC neurons project to the second order neurons in the paraventricular nucleus (PVN) but also to the lateral hypothalamus (LH), the ventromedial hypothalamus (VMN) and the dorsomedial hypothalamus (DMH) [96]. The PVN is responsible, in part, for the secretion of a wide range of regulatory neuropeptides and by the control of sympathetic nervous system activity to peripheral organs [18]. After a meal, POMC is cleaved in α-melanocyte- stimulating hormone (α-MSH) that is released by the POMC neurons to activate melanocortin 3 and 4 receptors (MC3/4R) in both hypothalamic and extra-hypothalamic neurons to suppress feeding and food intake and stimulate energy expenditure [97]. In general, insulin and leptin, by acting on POMC neurons, suppress feeding and promote energy expenditure (Figure 2). In fact, these hormones in POMC neurons also have a role in the regulation of peripheral glucose homeostasis, although the mechanisms involved are not so clear. The disruption of hypothalamic insulin and leptin pathways in POMC neurons, driven by insulin and leptin receptors deletion in these neurons, leads to a state of systemic insulin resistance and deterioration of glucose homeostasis [18]. This suggests that intact insulin and leptin hypothalamic pathways are required for a correct peripheral insulin and glucose homeostasis.
In contrast, in AgRP/NPy neurons, insulin regulates hepatic glucose production by inducing hyperpolarization and decreasing firing between AgRP neurons, which leads to reduced gluconeogenesis. Also, leptin directly acts on AgRP neurons at the hypothalamus to exert an inhibitory effect. As such, the crucial effect of leptin in the hypothalamus is to promote energy expenditure and inhibit food intake [18,97].
Apart from the regulation of feeding behavior and glucose homeostasis, insulin has been shown to have other functions in the brain as the regulation of cognitive functions, particularly memory. Notably, defects in insulin signaling in the brain may contribute to neurodegenerative disorders and damage of the cognitive system leading to dementia states.
2. Insulin and Cognitive Function
The role of insulin in the brain was first studied in the context of energy homeostasis, a process mainly regulated by the hypothalamus [68]. More recently, the role of insulin in other brain functions such as memory and cognition, neuronal development, and plasticity have been explored [6,98]. Insulin has a key role in the hippocampus, a brain region that expresses high levels of IR and that is involved in learning and memory [99].
The hippocampus is a complex structure in the brain located in the temporal lobe. It forms part of the limbic system, being an extension of the cerebral cortex [100]. The hippocampus is a very plastic region, with a key role in memory formation, organization, and storage [100]. The hippocampus converts short-term memory into long-term memory, solves spatial memory, and recollects the past experiences of places. It also plays a pivotal role in emotions and behavior [101]. Different parts of the hippocampus have distinct functions in certain types of memory: spatial memory, mainly processed by the rear part of the hippocampus; memory consolidation, a process by which the hippocampus organizes the stored information in the neocortex; and memory transfer, since long term memories are not stored in the hippocampus [101]. Information in the hippocampus travels along a unidirectional trisynaptic circuit originating from the entorhinal cortex and projecting to the dentate gyrus (DG), then to area CA3, and finally to area CA1 of the horn of Ammon [102,103]. These areas of the hippocampus have different functions, being composed of different types of neurons: the granular cells in the DG and pyramidal cells in the areas CA1 and CA3 of the horn of Ammon with a vast network of interneurons [102]. Special attention has been paid to the CA3 region for its specific role in memory formation and neurodegeneration [104]. CA3 receive excitatory inputs from the pyramidal cells and then inhibitory feedback that inhibit the pyramidal cells. This recurrent inhibition is a simple feedback circuit that can dampen excitatory responses in the hippocampus, being involved in memory formation processes [105,106].
The hippocampus has also an important role in brain plasticity. Brain plasticity underlies learning and memory and depends both on the activity and the number of synapses. Synapses may be modulated via potentiation or depression, processes that regulate the formation of new dendritic spines promoting tasks, learning, and consolidating behavioral alterations [6,107]. It has been postulated that insulin may be involved in the regulation of synaptic plasticity mechanisms and memory formation, since the mRNA levels of IR in dendrites and synapses at the hippocampus is high [99,108]. In fact, hippocampal neurons treated with insulin for 48 h show an increase in the frequency of miniature excitatory postsynaptic currents (mEPSCs), whilst downregulation of IR with short hairpin RNAs (ShRNAs) leads to the formation of few dendritic spines and therefore to reduced frequency of mEPSCs [6,109]. Insulin was also shown to regulate neuronal plasticity by controlling long-term potentiation (LTP) and long-term depression (LTD) [110]. Moreover, IR substrate p53 (IRSp53) interacts with the postsynaptic density protein 95 (PSD-95), present at excitatory synapses, therefore regulating a variety of receptors and channels, and increasing dendritic spine formation [6,111]. Insulin further plays a role on glutamatergic response by increasing the recruitment of the N-methyl-D-aspartate receptors (NMDARs) to the membrane and by enhancing the phosphorylation of NR2A and NR2B subunits [112]. In agreement, IRS2 knockout mice showed lower activation of NR2B subunits and a decrease in the LTP at CA3-CA1 synapses, however with higher density of CA1 dendritic spines [112,113,114]. Additionally, downregulation of a-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (AMPARs) activity of CA1 neurons in the hippocampus is crucial to insulin-induced LTD, an important feature to process memory formation [115].
The hippocampus is not the single player in the generation and regulation of memory and cognition. The prefrontal cortex is known to mediate decision making being involved in the retrieval of remote long-term memory and supporting memory and consolidation in a time scale ranging from seconds to days [116]. In fact, the hippocampus and prefrontal cortex work together to support the rapid encoding of new information, consolidation, and organization of memory networks. The cerebral cortex is known to process information about objects and events that we experience, and about the places where they occurred. Additionally, the ventral hippocampus (in the rat) and the anterior hippocampus (in humans) sends information to the medial prefrontal cortex (mPFC), suggesting that the mPFC could accumulate interrelated memories. The information of mPFC is sent back to other cortical regions, however the mPFC may bias or select the retrieval of event information in the ‘what’ stream. Therefore, interactions between these two brain regions can support the ability to create contextual representations that are associated with recent memories and use them to remember memories that are appropriate within a given context [117].
Considering the key role of the cortex in memory and decision making, it was reported that patients with frontal lobe damage exhibit inappropriate social behaviors and memory decline [89], suggesting that these dysfunctions can result from an impairment of memory storage in the prefrontal cortex. In fact, insulin has also an important role in the cortex, specially the IGFs since they are involved in the process of dendritic elaboration and to the stimulation of neurite outgrowth from dissociated neurons [118].
Therefore, the impairment of insulin signaling in the brain will have a negative impact in some neuronal functions additionally to the ones related with metabolism. These defects in insulin action in the CNS and specially in the hippocampus and prefrontal cortex represent a possible relationship between metabolic and cognitive disorders [119,120].
This entry is adapted from the peer-reviewed paper 10.3390/nu14071425
This entry is offline, you can click here to edit this entry!
