Cutaneous adverse drug reaction (CADR) is common in both inpatient and outpatient clinical settings and has been associated with a large variety of medications. Drug reactions represent a significant burden to the healthcare system due to increased hospital stay durations and associated costs. Moreover, some of these reactions may be life-threatening. The most common clinical manifestation of a CADR is a maculopapular drug eruption (MDE). Due to its many clinical mimics and associations with a variety of histopathologic patterns, maculopapular drug eruption is difficult to definitively diagnose from both a clinical and histopathological perspective. Histopathologic criteria for the diagnosis of MDE, while not entirely specific, may aid in establishing a differential that includes a drug eruption. Suggestive features include epidermal spongiosis, mild lymphocytic infiltrate, and occasional necrotic keratinocytes; interface change at the DEJ; superficial perivascular and interstitial lymphocytic with or without eosinophils and neutrophils in the mid-to-deep dermis and mild papillary dermal edema; and dilation of superficial dermal lymph and blood vessels. Moreover, it is important to emphasize that a drug eruption should always be considered in the differential when multiple histopathologic patterns—none of which conform to another precise diagnosis—are present within the same tissue section. Thus, a biopsy can be a helpful diagnostic tool when MDE is suspected by demonstrating findings suggestive of MDE or by ruling out clinical mimics. However, biopsy results cannot be used in isolation as clinical-pathologic correlation is paramount in MDE.
- maculopapular eruption
- drug eruption
- dermatopathology
1. Introduction
2. Pathophysiology
3. Risk Factors
4. Differential Diagnosis
5. Histopathologic Features of Maculopapular Drug Eruption
5.1. Epidermal Features
5.2. Dermal-Epidermal Junction Features
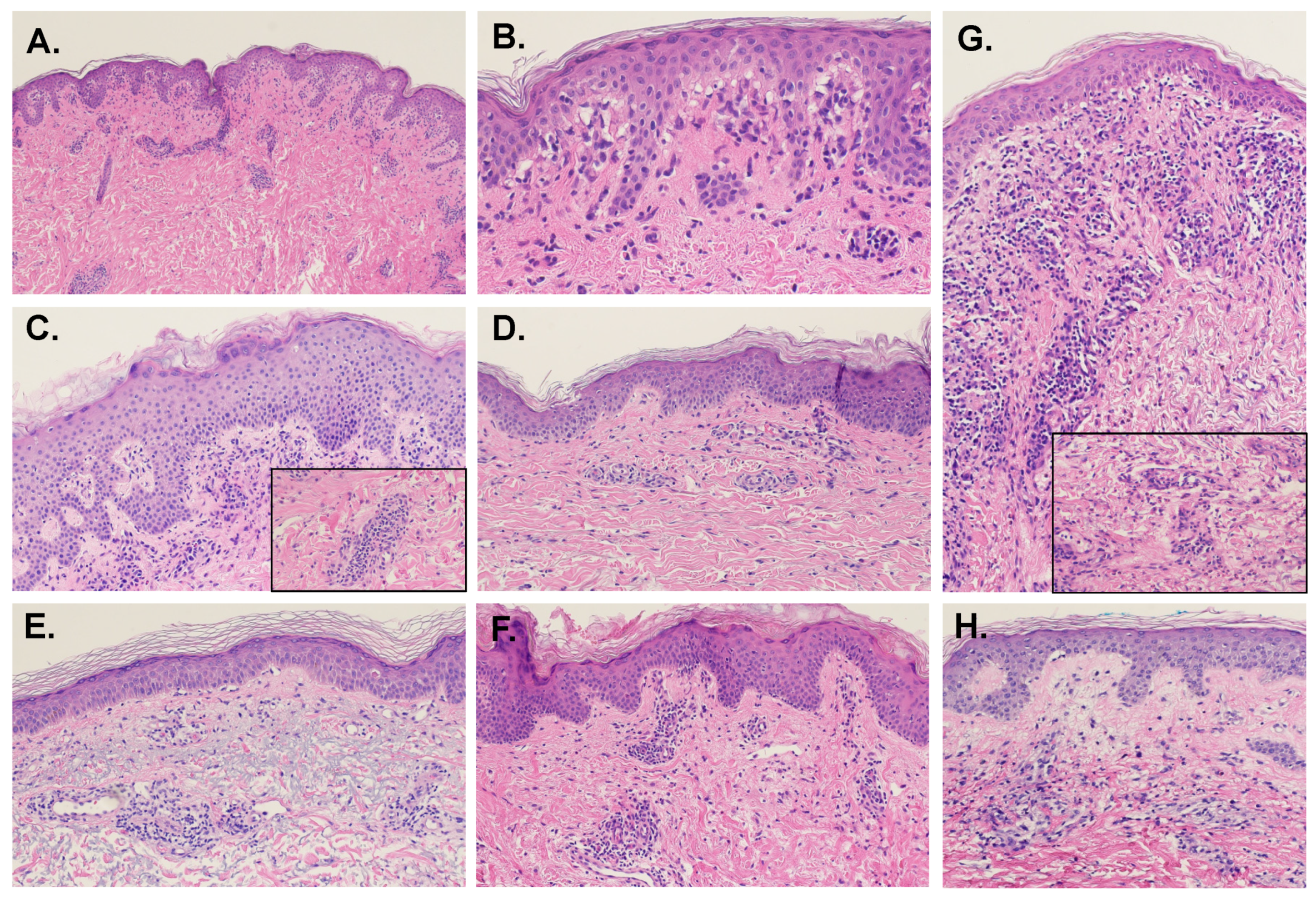
5.3. Dermal Features
5.4. Connective Tissue and Vasculature
5.5. Presence of Eosinophils
6. Variants of Maculopapular Drug Eruption
6.1. MDE with Urticarial Aspect
6.2. MDE with Granulomatous Change
6.3. MDE Due to Targeted and Other Oncologic Therapies
This entry is adapted from the peer-reviewed paper 10.3390/dermatopathology9020014
References
- Ellis, A.; Billings, S.D.; Khanna, U.; Warren, C.B.; Piliang, M.; Vij, A.; Ko, J.S.; Bergfeld, W.F.; Fernandez, A.P. Diagnoses of hospitalized patients with skin abnormalities prompting biopsy by consulting dermatologists: A 3-year review from a tertiary care center. J. Cutan. Pathol. 2020, 47, 346–356.
- Liao, P.; Shih, C.; Mao, C.; Deng, S.; Hsieh, M.; Hsu, K. The cutaneous adverse drug reactions: Risk factors, prognosis and economic impacts. Int. J. Clin. Pract. 2013, 67, 576–584.
- Roujeau, J.C.; Stern, R.S. Severe adverse cutaneous reactions to drugs. N. Engl. J. Med. 1994, 331, 1272–1285.
- Apaydin, R.; Bilen, N.; Dökmeci, S.; Bayramgürler, D.; Yildirim, G. Drug eruptions: A study including all inpatients and outpatients at a dermatology clinic of a university hospital. J. Eur. Acad. Derm. Venereol. 2000, 14, 518–520.
- Bigby, M.; Jick, S.; Jick, H.; Arndt, K. Drug-induced cutaneous reactions. A report from the Boston Collaborative Drug Surveillance Program on 15,438 consecutive inpatients, 1975 to 1982. JAMA 1986, 256, 3358–3363.
- Weyers, W.; Metze, D. Histopathology of drug eruptions—general criteria, common patterns, and differential diagnosis. Derm. Pract. Concept. 2011, 1, 33–47.
- Ukoha, U.T.; Pandya, A.G.; Dominguez, A.R. Morbilliform Drug Eruptions. In Cutaneous Drug Eruptions: Diagnosis, Histopathology and Therapy; Hall, J.C., Hall, B.J., Eds.; Springer: London, UK, 2015; pp. 45–53.
- Stern, R.S. Exanthematous Drug Eruptions. N. Engl. J. Med. 2012, 366, 2492–2501.
- Pichler, W.J.; Naisbitt, D.J.; Park, B.K. Immune pathomechanism of drug hypersensitivity reactions. J. Allergy Clin. Immunol. 2011, 127, S74–S81.
- Bronnimann, M.; Yawalkar, N. Histopathology of drug-induced exanthems: Is there a role in diagnosis of drug allergy? Curr. Opin. Allergy Clin. Immunol. 2005, 5, 317–321.
- Czarnobilska, E.; Obtułowicz, K.; Wsołek, K. . Przegl. Lek. 2007, 64, 506–508.
- Formica, D.; Sultana, J.; Cutroneo, P.M.; Lucchesi, S.; Angelica, R.; Crisafulli, S.; Ingrasciotta, Y.; Salvo, F.; Spina, E.; Trifirò, G. The economic burden of preventable adverse drug reactions: A systematic review of observational studies. Expert Opin. Drug Saf. 2018, 17, 681–695.
- Neuman, M.G.; McKinney, K.K.; Nanau, R.M.; Kong, V.; Malkiewicz, I.; Mazulli, T.; Moussa, G.; Cohen, L.B. Drug-induced severe adverse reaction enhanced by human herpes virus-6 reactivation. Transl. Res. 2013, 161, 430–440.
- Pritchett, J.C.; Nanau, R.M.; Neuman, M.G. The Link between Hypersensitivity Syndrome Reaction Development and Human Herpes Virus-6 Reactivation. Int. J. Hepatol. 2012, 2012, 723062.
- Hung, S.-I.; Chung, W.-H.; Jee, S.-H.; Chen, W.-C.; Chang, Y.-T.; Lee, W.-R.; Hu, S.-L.; Wu, M.-T.; Chen, G.-S.; Wong, T.-W.; et al. Genetic susceptibility to carbamazepine-induced cutaneous adverse drug reactions. Pharm. Genom. 2006, 16, 297–306.
- Naim, M.; Weyers, W.; Metze, D. Histopathologic features of exanthematous drug eruptions of the macular and papular type. Am. J. Derm. 2011, 33, 695–704.
- Kantor, E.D.; Rehm, C.D.; Haas, J.S.; Chan, A.T.; Giovannucci, E.L. Trends in Prescription Drug Use Among Adults in the United States From 1999–2012. JAMA 2015, 314, 1818–1831.
- Singh, S.; Khandpur, S.; Arava, S.; Rath, R.; Ramam, M.; Singh, M.; Sharma, V.K.; Kabra, S.K. Assessment of histopathological features of maculopapular viral exanthem and drug-induced exanthem. J. Cutan. Pathol. 2017, 44, 1038–1048.
- Naranjo, C.A.; Busto, U.; Sellers, E.M.; Sandor, P.; Ruiz, I.; Roberts, E.; Janecek, E.; Domecq, C.; Greenblatt, D. A method for estimating the probability of adverse drug reactions. Clin. Pharmacol. Ther. 1981, 30, 239–245.
- Ardern-Jones, M.R.; Mockenhaupt, M. Making a diagnosis in severe cutaneous drug hypersensitivity reactions. Curr. Opin. Allergy Clin. Immunol. 2019, 19, 283–293.
- Valks, R.; Vargas, E.; Muñoz, E.; Fernández-Herrera, J.; Garcia-Diéz, A.; Fraga, J. Dermal infiltrate of enlarged macrophages in patients receiving chemotherapy. J. Cutan. Pathol. 1998, 25, 259–264.
- Bellini, V.; Pelliccia, S.; Lisi, P. Drug- and Virus- or Bacteria-induced Exanthems: The Role of Immunohistochemical Staining for Cytokines in Differential Diagnosis. Dermatitis 2013, 24, 85–90.
- Gerson, D.; Sriganeshan, V.; Alexis, J.B. Cutaneous drug eruptions: A 5-year experience. J. Am. Acad. Dermatol. 2008, 59, 995–999.
- Nghiem, P. The “drug vs graft-vs-host disease” conundrum gets tougher, but there is an answer: The challenge to dermatologists. Arch. Derm. 2001, 137, 75–76.
- Billings, S.; Cotton, J. Inflammatory Dermatopathology: A Pathologist’s Survival Guide; Springer Science+Business Media, LLC: Berlin, Germany, 2011.
- Weinborn, M.; Barbaud, A.; Truchetet, F.; Beurey, P.; Germain, L.; Cribier, B. Histopathological study of six types of adverse cutaneous drug reactions using granulysin expression. Int. J. Dermatol. 2016, 55, 1225–1233.
- Curry, J.L.; Torres-Cabala, C.A.; Kim, K.B.; Tetzlaff, M.T.; Duvic, M.; Tsai, K.Y.; Hong, D.S.; Prieto, V.G. Dermatologic toxicities to targeted cancer therapy: Shared clinical and histologic adverse skin reactions. Int. J. Dermatol. 2014, 53, 376–384.
- Marra, D.E.; McKee, P.H.; Nghiem, P. Tissue eosinophils and the perils of using skin biopsy specimens to distinguish between drug hypersensitivity and cutaneous graft-versus-host disease. J. Am. Acad Derm. 2004, 51, 543–546.
- Weaver, J.; Bergfeld, W.F. Quantitative analysis of eosinophils in acute graft-versus-host disease compared with drug hypersensitivity reactions. Am. J. Derm. 2010, 32, 31–34.
- Stevens, A.; Dalziel, K. The histopathology of drug rashes. Curr. Diagn. Pathol. 1998, 5, 138–149.
- Horn, T.D.; Burke, P.J.; Karp, J.E.; Hood, A.F. Intravenous Administration of Recombinant Human Granulocyte-Macrophage Colony-Stimulating Factor Causes a Cutaneous Eruption. Arch. Dermatol. 1991, 127, 49–52.
- Alvarez-Ruiz, S.; Penas, P.; Fernández-Herrera, J.; Sánchez-Pérez, J.; Fraga, J.; García-Díez, A. Maculopapular eruption with enlarged macrophages in eight patients receiving G-CSF or GM-CSF. J. Eur. Acad. Dermatol. Venereol. 2004, 18, 310–313.
- Macdonald, J.B.; Macdonald, B.; Golitz, L.E.; LoRusso, P.; Sekulic, A. Cutaneous adverse effects of targeted therapies: Part I: Inhibitors of the cellular membrane. J. Am. Acad. Derm. 2015, 72, 203–218.
- Ransohoff, J.D.; Kwong, B.Y. Cutaneous adverse events of targeted therapies for hematolymphoid malignancies. Clin. Lymphoma Myeloma Leuk. 2017, 17, 834–851.
- Sinha, R.; Edmonds, K.; Newton-Bishop, J.A.; Gore, M.E.; Larkin, J.; Fearfield, L. Cutaneous adverse events associated with vemurafenib in patients with metastatic melanoma: Practical advice on diagnosis, prevention and management of the main treatment-related skin toxicities. Br. J. Derm. 2012, 167, 987–994.
- Rossini, M.; De Souza, E.; Cintra, M.; Pagnano, K.; Chiari, A.; Lorand-Metze, I. Cutaneous adverse reaction to 2-chlorodeoxyadenosine with histological flame figures in patients with chronic lymphocytic leukaemia. J. Eur. Acad. Dermatol. Venereol. 2004, 18, 538–542.
- Soefje, S.A.; Karnad, A.; Brenner, A.J. Common toxicities of mammalian target of rapamycin inhibitors. Target. Oncol. 2011, 6, 125–129.
- Raymond, E.; Alexandre, J.; Faivre, S.; Vera, K.; Materman, E.; Boni, J.; Leister, C.; Korth-Bradley, J.; Hanauske, A.; Armand, J.-P. Safety and pharmacokinetics of escalated doses of weekly intravenous infusion of CCI-779, a novel mTOR inhibitor, in patients with cancer. J. Clin. Oncol. 2004, 22, 2336–2347.
- Prieto-Torres, L.; Llamas-Velasco, M.; Machan, S.; Haro, R.; De Asis, S.; Carmo, M.; Loredo, A.; Del Puerto, C.; Fried, I.; Kempf, W. Taxanes-induced cutaneous eruption: Another histopathologic mimicker of malignancy. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 638–644.
