Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Angiogenesis is a key process in various physiological conditions in the nervous system and in the retina during postnatal life. Although an increasing number of
studies have addressed the role of endothelial cells in this event, the astrocytes contribution in angiogenesis has received less attention.
- brain angiogenesis
- retinal angiogenesis
- astrocytes
- scaffold
1. Introduction
Blood vessel growth during early postnatal brain development requires an increase in progenitor cells and their differentiation into astrocytes in the central nervous system [1]. Strong coordination between the astrocyte differentiation and the vessel growth during this stage of brain development has been shown and, in fact, angiogenesis (process where new blood vessels are formed from pre-existing vessels) and astrogenesis occur almost at the same time [2]. In vitro studies have confirmed the relevance of astrocyte differentiation for the development of brain vasculature and the importance of establishing co-culture models of astrocytes and endothelial cells (ECs) to study interactions between these cells during angiogenesis [3].
Specific genetic inhibition of astrogliogenesis in the early postnatal mouse cortex resulted in an important delay in blood vessel growth and branching, which is consistent with the critical role of astrocytes in early postnatal brain development [4]. Moreover, astrogliosis, a complex process with positive and negative effects associated with the expression of many genes and morphological changes in astrocytes when an insult in the central nervous system occurs, has been shown to be necessary to restore blood vessel morphology by increasing proangiogenic signaling mediated by astrocytes, but negative effects have also been described [1][4]. Several studies have been focused on determining the role of astrocytes in the angiogenic process from a mechanistic point of view. Many of these studies have used the murine retina, an excellent model to study the signaling related to angiogenesis in the central nervous system, particularly the signals released from astrocytes during this process, due its easy anatomical accessibility. Angiogenesis seems to depend on the communication between different cells, such as neurons, glia, ECs, pericytes, and even immune cells in the brain and in the retinal angiogenesis [5][6][7][8]. These studies show the relevance of the astrocytes in the angiogenesis process during the brain and in retina development angiogenesis, which seems to have similarities regarding the complex mechanisms involved, and where the astrocytes play a key role.
2. Astrocytes as Templates for Angiogenesis
Astrocytes are the most abundant cell type in the brain; they appear at the later stages of brain development, with maximum astrogenesis in the first postnatal days [9][10]. According to this, astrocytes have an important role in angiogenesis during postnatal development and participate in the consolidation of the primary vasculature in the brain, acting as a template over which ECs migrate to form new blood vessels [4][9]. In addition to the relevance of the astrocytes in the brain angiogenesis process, in numerous studies using the mouse retinal model, researchers have shown that astrocyte scaffolding functions as a template for vascular network development in the retina [11][12][13]. In fact, the disruption of the angiogenic astrocyte template induces alterations in the retinal angiogenic process [14]. Similar to what was observed in the brain, Phng et al. reported that during a three-week period after birth, ECs migrate over a preexisting astrocytic template and form new blood vessels in the mouse retina [15].
Various cell adhesion molecules as integrins, cadherins and laminin protein complexes from astrocytes, have been associated with their role as scaffolding for angiogenesis, and their role will be described below.
2.1. Cadherins: A Possible Role as an Angiogenic Cue
Cadherins are a calcium-dependent family of transmembrane proteins with an important function in cell to cell adhesion, awarding stability and mechanical resistance, and playing an important role in the morphogenesis and homeostasis of tissue [16]. There have been described different types of classical cadherins, named according their first reported localization despite that they are not exclusively expressed in that tissue: N-cadherin (neural), P-cadherin (placenta), T-cadherin (heart), VE-cadherin (vascular epithelial), and R-cadherin (retinal) [17], but researchers will focus in some which are expressed in astrocytes and would be related with the angiogenesis process in the brain and retina.
Perinatal astrocytes and oligodendrocytes express N-cadherin on their surfaces, and the blockade of N-cadherin increases the migration of oligodendrocytes on an astrocyte cell surface, suggesting that the cell interaction mediated by this cadherin has a negative effect on cell migration [18]. Moreover, it has been described that endothelial signaling through N-cadherin reduces the polarized phenotype of migrating smooth muscle cells, suggesting a role in blood vessel stabilization [19].
In addition, R-cadherin has shown an important function in retinal morphogenesis, and is expressed in glial cells and in early optic nerve glia in the rat optic nerve, which is an immature kind of astrocyte [20]. It has been demonstrated that N-cadherin axons elongate on using glial R-cadherins as substrate [21]. During the postnatal stage, R-cadherin expression is higher between postnatal days 0 to 4 (P0 to P4) than postnatal day 8 (P8) in the retinal astrocytic process surrounding new vessels, and coincides spatially and temporarily with the formation of the vascular network. This suggests that small R-cadherin clusters represent sites of endothelial filopodial extension. The blockade of R-cadherin prevented the normal extensive collateralization in the superficial vessel network and had negative effects in the deep vessel network [22], and this could indicate that R-cadherin from the astrocytes template serves as a cue for ECs migration during angiogenesis.
An atypical cadherin, FAT1 cadherin, is expressed in the nervous system with an important function in neural differentiation [23], and also in the regulation of cell polarity and migration [24]. FAT1, and one of its signaling pathways, Hippo, have been associated with the early stages of neurogenesis, but their regulation is not completely understood [23]. In addition, Fat1 is also expressed in retinal astrocytes during postnatal life [25]. Consistent with that, the elimination of Fat1 in postnatal retinas impaired the association of ECs and astrocytes, proliferation and migration of astrocytes progenitors, and their maturation into immature astrocytes, and delays postnatal angiogenesis [25]. This is in line with a previous report from Caruso et al. (2013), where Fat1 mutant mice displayed retinal microvascular abnormalities as microaneurysms [26]. However, if this cadherin is involved in angiogenesis in the brain, it has not been addressed.
In summary, cadherins such as R-cadherin and the atypical Fat1 cadherin promote angiogenesis in the retina, and N-cadherin seems to be relevant for blood vessel stabilization. Future studies are needed to characterize the signaling pathways between ECs and astrocytes mediated by cadherins during angiogenesis.
2.2. Fibronectin and Integrins in Scaffold Formation
Fibronectin is an important component of the extracellular matrix which permits cell adhesion through the transmembrane receptors named integrins, participating in the cell proliferation process [27].
Astrocytes are the major source of fibronectin during retinal angiogenesis. A study conducted by Stenzel et al. supports the idea that fibronectin secreted by astrocytes provides a scaffold for guiding directional angiogenesis into the avascular region of the retina, and the key role of this mechanism during the retinal angiogenic process [28]. In fact, the specific deletion of fibronectin from astrocytes resulted in not only a reduction in ECs migration, but also a reduced signaling through phosphatidylinositol 3-kinase/protein kinase B or PKB (PI3K/Akt signaling), and the vascular endothelial growth factor receptor-2 (VEGFR-2) expression [28]. PI3K is activated by growth factors such as endothelial cell growth factor (VEGF), which is a recognized angiogenic molecule in physiological and physiopathological conditions [29]. The binding of VEGF to VEGFR2 in ECs, activates the downstream kinase Akt to promote angiogenesis by activating mammalian target of rapamycin (mTOR). This pathway may have a key role in the proliferation, adhesion, and migration of ECs during the angiogenic process [30][31]. In accordance with the relevant role of fibronectin in angiogenesis, the inhibition of fibronectin production from astrocytes decreased the activation of VEGFR2 and consequently PI3K/Akt signaling, which had negative effects on filopodia adhesion to the extracellular matrix and tip cell migration; as result, the radial expansion of the vascular plexus was reduced [28].
During normal retinal development, the high cell proliferation rate produces physiological hypoxia that activates hypoxia inducible factors (HIF), which induce angiogenesis to ensure the appropriate oxygen levels and nutrients in the developing tissues [32][33]. Astrocytes may act as oxygen sensors, and hypoxia during retinal development could induce astrocyte proliferation to establish an adequate template for retinal angiogenesis [14]. In this context, the orphan nuclear receptor tailless (TLX) is one of the mediators downstream of HIF signaling produced by astrocytes that has an important role during retinal development [34]. TLX (NR2E1), a nuclear receptor that acts as a transcription factor, is predominantly expressed in the central nervous system, and has a critical role in the embryonic and adult neurogenic process and neural development, especially in the visual system [35][36][37]. Studies from Uemura et al. showed that TLX expression increases in proangiogenic astrocytes and has a crucial role in the formation of the extracellular scaffold formed by these astrocytes through the upregulation of the expression and extracellular deposition of fibronectin, which provides the optimal conditions for survival, adhesion, and migration of ECs [34]. In fact, genetic ablation of Tlx abrogated normal retinal vascular development, as evidenced by deficiencies in the deposition of fibronectin, which produced a disorganized architecture of the astrocyte network and abnormal angiogenesis during the postnatal stage in this model. Additionally to the fibronectin deposition surrounded astrocytes networks dependent of Tlx signaling, an increased α5 and β1 integrin subunits expression in ECs was observed, which is consistent a role of fibronectin/integrin signaling in the adhesion of migrating ECs to the astrocytic scaffold during the development of the retinal vascular system [35]. Moreover, the binding of angiopoietin-1 (Ang-1), a secreted glycoprotein that is part of the angiopoietin family of growth factors, has an important role in vascular development, remodeling, and stabilization, through its binding to tyrosine kinase receptors in ECs [38]. It is known that Ang-1 is secreted by astrocytes in hypoxic conditions (see below), and it activates the αvβ5-focal adhesion kinase (FAK)–AKT signaling pathway in retinal astrocytes and stimulates fibronectin secretion [39]. These antecedents suggest that hypoxia via Tlx pathway or/and Ang-1 pathway promote the fibronectin secretion from astrocytes that later interacts with integrins in ECs promoting retinal angiogenesis.
Furthermore, to the role of fibronectin in the retinal angiogenesis, it has been reported that fibronectin is essential for angiogenesis in the brain, promoting the survival and also the proliferation of capillaries ECs by the activation of the mitogen-activated protein kinase (MAPK) signaling pathway through integrin receptors α5β1 and αvβ3 [40]. These observations are consistent with the overexpression of fibronectin and its receptors α5β1 and αvβ3 in angiogenic vessels, as response to an ischemic event in the brain in murine models [41][42]. This suggests that similar to what occurs during retinal angiogenesis, the interaction between integrins and fibronectin during hypoxia stimulates brain angiogenesis.
Consistent with the role of integrins in angiogenesis, the correct retinal vascular plexus formation in the mouse retina has been shown to be sensitive to the ablation of the αvβ8 integrin expressed in retinal astrocytes, evidenced by the impairment of the development of the vascular plexus and a significantly lower number and length of filopodia in tip ECs. This phenomenon was related to the lack of activation of the transforming growth factor β (TGFβ) signaling in ECs and highlights the importance of the αvβ8 integrin from astrocytes in the control of retinal angiogenesis [12]. In contrast with these findings, in cocultures of astrocytes and brain ECs, the astrocytic αvβ8 activation was dependent of TGFβ, and a possible signaling through their receptors induced the expression of two known antiangiogenic molecules in ECs, the plasminogen inhibitor-1 and thrombospondin-1, and inhibited endothelial migration [43]. This suggests that in the brain, the αvβ8 integrin-TGFβ pathway has a role in blood vessel maturation and stabilization.
2.3. Laminins as a Template for Angiogenesis
According to the relationship of the extracellular matrix to astrocyte signaling during angiogenesis in retinal models, the loss of other component of the extracellular matrix, as laminin, and integrin, as was previously described, also had negative effects on the retinal angiogenic process [44][45].
Laminins are a glycoprotein family that are present in the extracellular matrix and are part of the basement membrane. These proteins can bind to their transmembrane receptors, the integrin family, expressed in many types of cells and mediate many processes in adhesion-mediated events in vertebrates [46]. Deletion of laminins β2 and γ3 in mouse models was shown to reduce astrocyte migration, probably through the reduction of astrocyte expression of β1 integrin, indicating the importance of laminin-integrin β1 interactions for laminin-directed astrocyte migration. Furthermore, deletion of laminins reduced the interaction between astrocytes and ECs and affected normal retinal blood vessels [44]. Moreover, it has been described that assembly of laminin network with proteoglycans are needed for the astrocytic migration and angiogenesis [47]. Further, laminin released by astrocytes seems to recruit and activate the microglia, and changes in the microglia contribute to angiogenesis in retinal models [45]. These results highlight the relevance of laminin-β1 integrin signaling and laminin-proteoglycan interaction to promote astrocyte migration and form the template for angiogenesis [44]. Future studies to elucidate the role of laminin in this process in depth greater are required.
The representation of the principal proteins (as fibronectin, cadherins, integrins, laminin, and others) and its receptors previously described, associated with astrocytes as templates for angiogenesis, is summarized in Figure 1.
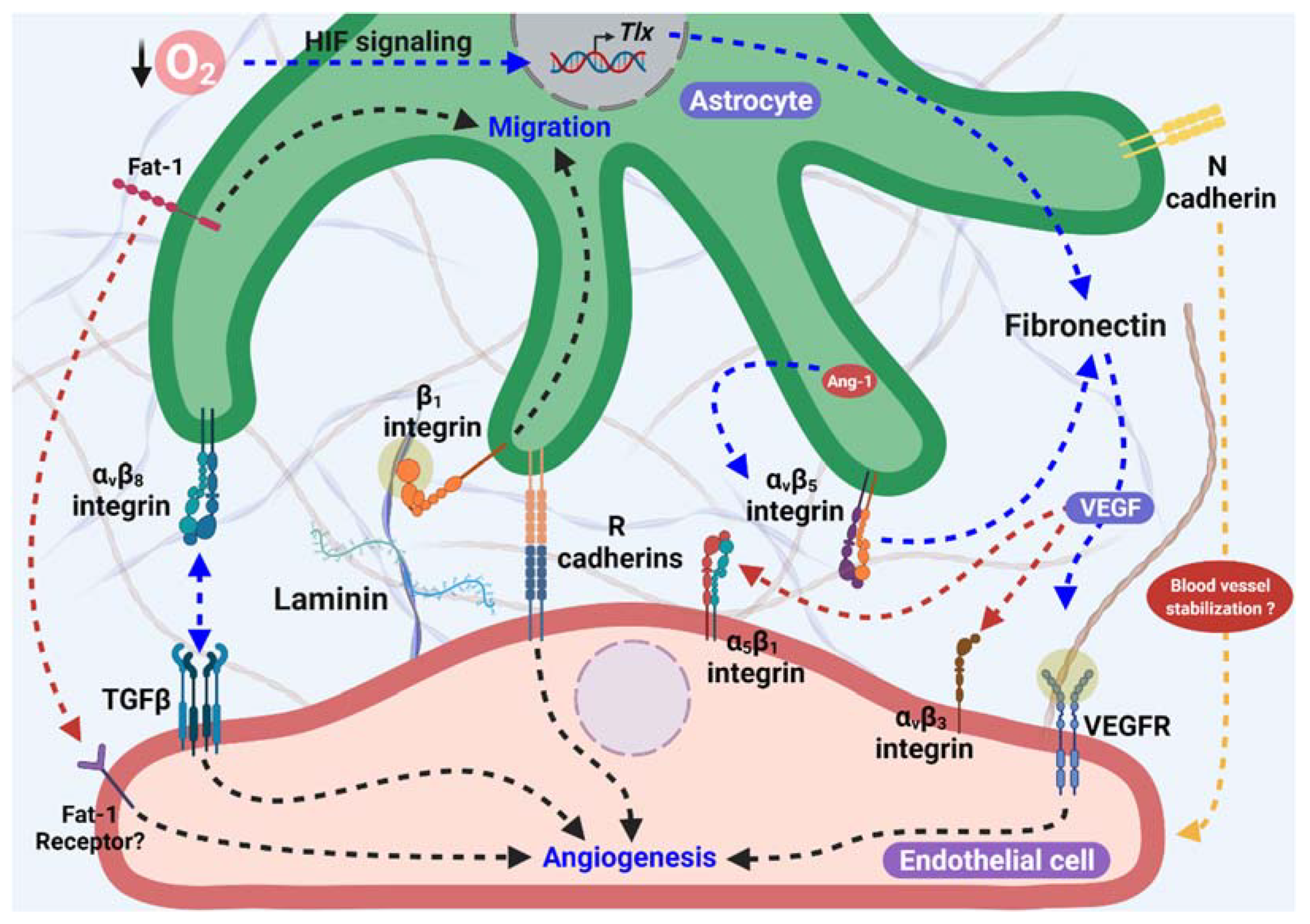 Figure 1. Astrocytes as proangiogenic scaffolds. R-cadherin in the astrocyte process surrounding the endothelial filopodia extension forms small clusters, and promotes angiogenesis. In addition, Fat-1 cadherin from astrocytes is necessary for endothelial cells (ECs)-astrocytes interaction, and also to proliferation and maturation of astrocyte progenitor cells in the astrocyte template. Furthermore, Fibronectin production in astrocytes is stimulated by lower O2 levels in avascular areas through HIF and TLX signaling and/or in an Ang-1 dependent manner, which activate α5β1 and αvβ3 integrins and VEGFR2 signaling, inducing endothelial migration. αvβ8 integrin from astrocytes is necessary for ECs migration dependent on the activation of TGFβ signaling. Additionally, laminin-β1 integrin induces astrocyte migration and promotes astrocyte scaffolding formation. Moreover, the participation of N-cadherin in blood vessel stabilization has been suggested.
Figure 1. Astrocytes as proangiogenic scaffolds. R-cadherin in the astrocyte process surrounding the endothelial filopodia extension forms small clusters, and promotes angiogenesis. In addition, Fat-1 cadherin from astrocytes is necessary for endothelial cells (ECs)-astrocytes interaction, and also to proliferation and maturation of astrocyte progenitor cells in the astrocyte template. Furthermore, Fibronectin production in astrocytes is stimulated by lower O2 levels in avascular areas through HIF and TLX signaling and/or in an Ang-1 dependent manner, which activate α5β1 and αvβ3 integrins and VEGFR2 signaling, inducing endothelial migration. αvβ8 integrin from astrocytes is necessary for ECs migration dependent on the activation of TGFβ signaling. Additionally, laminin-β1 integrin induces astrocyte migration and promotes astrocyte scaffolding formation. Moreover, the participation of N-cadherin in blood vessel stabilization has been suggested.In addition to the participation of the extracellular matrix proteins and cell adhesion molecules described above, various studies have focused on the mediators released from the astrocytes that contribute to the angiogenic process during brain development and in the retina.
This entry is adapted from the peer-reviewed paper 10.3390/ijms23052646
This entry is offline, you can click here to edit this entry!
