Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Physiology
Galectin-3 (Gal-3) is a 30KDa lectin implicated in multiple pathophysiology pathways including renal damage and fibrosis. Gal-3 binds β-galactoside through its carbohydrate-recognition domain.
- galectin-3
- kidney disease
- lectins
1. Gal-3: A Carbohydrate Binding Protein from the Lectin Family
1.1. The Lectin Family
The term “lectin” is derived from the Latin word “legere” which means “to collect” and defines a group of binding proteins that interact with multiple partners via a specific carbohydrate recognition domain (CRD) [4]. Because of these interactions, lectins are implicated in a wide variety of pathways (e.g., from transduction pathway to cell-to-cell interaction) [5,6].
Galectins are a 14-member family of proteins within the lectins that bind β-galactose via their specific CRD. Galectins are divided into three groups, depending on their CRD and protein structures: galectins 1, 2, 5, 7, 10, 11, 13, 14, 15, and 16 are composed of one unique CRD associated in monomer or in dimer; galectins 4, 6, 8, 9, and 12 are composed of two different united CRDs; and Gal-3 is a chimeric protein including one CRD and one regulatory N-terminal domain connected with a collagen-like sequence [7]. Galectins’ architecture is described in Figure 1a. Galectins are involved in various biological processes such as early development, cell migration, immunological signaling leading to profibrotic effects [8,9,10], and cell-to-cell communication [11].
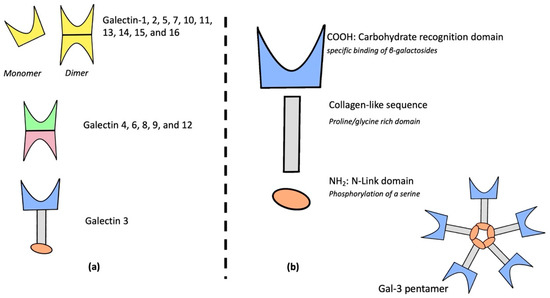
Figure 1. Galectins’ family structure (a), galectin-3 chimeric and specific structure (b).
2. Gal-3 in Preclinical Models of Kidney Disease
The role of Galectin-3 is summarized in Figure 3.
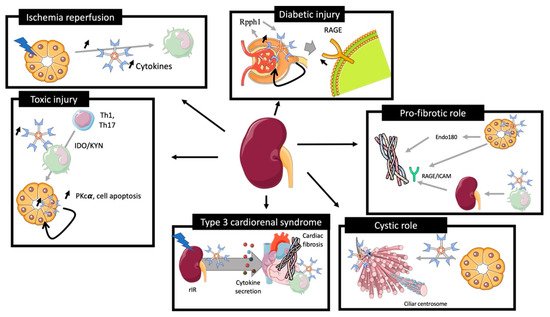
Figure 3. Role of galectin-3 in preclinical models of kidney injury. rIR leads to an increase of plasma and renal Gal-3 expression associated with acute tubular injury, promoting cytokine expression and immune cells recruitment. Toxic renal injury induces Gal-3 associated Th1 and Th17 recruitment for renal reparation and Gal-3 tissular associated PKcα
2.1. Ischemia/Reperfusion
Nishiyama et al. showed that rIR in rats induced a rapid Gal-3 mRNA overexpression and had a negative correlation with serum creatinine dosage at 48 h (R = −0.94). Gal-3 overexpression was extended in distal tubules 48 h after the injury [59]. Furthermore, genetic inhibition of Gal-3 in this model was associated with less macrophage infiltration and activation, less tissue damage (ROS production, tubular necrosis), and improved renal function [60].
2.2. Toxic Injury
In toxic preclinical models of experimental nephropathy, tubular damage appears less pronounced than in rIR models. In a toxic model of nephropathy induced by folic acid (FA), Nishiyama et al. observed a rapid increase of renal Gal-3 mRNA expression [59]. Furthermore, a spread expression of Gal-3 from proximal ducts to a diverse subset of tubules, including dilated collecting ducts, was observed. Fourteen days after injury, Gal-3 was also detected in macrophages and fibrosis progression was prevented after Gal-3 inhibition with less renal apoptosis and inflammation [64].
In a cisplatin-induced AKI model, Li et al. observed an increase of Gal-3 renal expression at day 3, associated with an overexpression of PKC-α, cell apoptosis, and collagen type I synthesis. Interestingly, Gal-3 inhibition limited the AKI to CKD transition [65]. In contrast, Volarevic et al. demonstrated, in a similar model of AKI, that Gal-3 expression in immune cells was associated with regulation of immunosuppression via renal dendritic cells, TLR-2 activation, and IL-10 secretion. These findings suggested a possible deleterious role of Gal-3 inhibition for immune regulation [66].
2.3. Glomerular Injury
Altered expression of Gal-3 within injured glomeruli has been reported in some studies. In a streptozotocin model of diabetic nephropathy in rats, Gal-3 was overexpressed from 2 to 12 weeks in diabetic rats, and mainly in mesangial cells. Overexpression of Gal-3 modulated the glomerular remodeling of associated advance-glycation-end-product (AGE) receptor (RAGE) [67]. Using the same experimental model, these authors demonstrated that Gal-3 deficiency resulted in accelerated diabetic glomerulopathy accompanied by glomerular AGE accumulation due to RAGE downregulation [68]. In addition, injection of N-carboxylmethyllysine in mice induced AGE accumulation associated with the glomerular injury. Furthermore, Gal-3 inhibition leads to an accelerated glomerular disease via higher circulating AGE levels and altered RAGE functions [69]. Zhang et al. proposed a mechanism of AGE-mediated damage via a long non-coding sequence Rpph1 interacting with Gal-3, promoting MERF/ERK transduction pathway resulting in MCP-1 overexpression and mesangial cell proliferation [70].
2.4. Immune-Associated Renal Damage
2.4.1. Sepsis-Associated Renal Disease
In a peritonitis model in rats (cecal ligature puncture), Gal-3 was upregulated in septic-associated renal damage. Furthermore, pharmacological inhibition of Gal-3 using modified citrus pectin (MCP) decreased IL-6 plasma and renal inflammation and improved renal function and survival [73].
2.4.2. Transplantation Model
Gal-3 has been shown for several years to modulate inflammation and immune cell infiltration in pathophysiological conditions. Grafted models are associated with immune cell-related graft dysfunction. Dang et al. grafted BM12 kidney from WT mice, in WT mice or in Gal-3 null mice. In WT mice, this graft resulted in Gal-3 tissular and plasmatic upregulation. Gal-3 null mice had less tubular injury, moderate fibrosis, and a reduced amount of immune cells infiltration [42]. These studies confirmed the role of Gal-3 in the recruitment of immune cells during the pathological context, and its inhibition improved renal outcome.
2.5. Polycystic Model
In a model of congenital polycystic kidney (CPK) in mice, Gal-3 expression was observed in cilia of dilated collecting ducts. Interestingly, injection of Gal-3 decreased the cyst number in mice whereas Gal-3 null mice showed a higher kidney weight/bone length ratio and a modified cilia structure.
2.6. Renal Fibrosis
Gal-3 has been reported to promote fibrosis in several organs [1] and plays a major role in the renal transition from acute to chronic disease, promoting inflammatory factors release, inflammatory cell activation, and tissue injury [75]. However, the role of Gal-3 in this purpose is still a matter of debate.
Gal-3 is associated with the proliferation of extra-cellular matrix-producing cells (fibroblast and myofibroblast), which may be in turn associated with the migration and adhesion of such cells [76].
It has also been reported that the degree of renal damage and fibrosis was more extensive in Gal-3 null mice with increased total collagen, whereas a corresponding decrease of myofibroblast and extra-cellular matrix synthesis via a downregulation of endo180 receptors implicated in collagen degradation was observed [77].
2.7. Preclinical Model of Cardio-Renal Syndrome
It was recently identified the potential role of Gal-3 in type 3 cardio-renal syndrome. In this study, mice were operated using left renal ischemia reperfusion after right nephrectomy to induce a transient renal dysfunction, rapidly normalized at 48 h, inducing no water overload or no uremic syndrome. This leads to a decrease of cardiac fraction shortening and an increase of cardiac fibrosis after 28 days. Similar results were observed after unilateral ureteral occlusion. This study highlighted the role of acute renal damage leading to a beginning of cardiac dysfunction. In this experimental model, Gal-3 was increased after renal ischemia reperfusion in plasma, renal tissue, and later in cardiac tissue. The inhibition of Gal-3 prevented cardiac dysfunction. Furthermore, we identified in a model of bone marrow graft mice that immune cells expressing Gal-3 in the heart promoted cardiac fibrosis and its inhibition in bone marrow grafted cells improved cardiac phenotype. This study suggests a potential role of Gal-3 in crosstalk between the kidney and the heart during type 3 cardio-renal syndrome [61]. The role of Gal-3 in the initiation of cardiorenal syndrome remains unknown, some preclinical studies are trying to identify the renal role of Gal-3 in this context [80].
3. Gal-3 as a Biomarker
3.1. Kidney Function
Drechsler et al. measured Gal-3 baseline level from the German Diabetes mellitus Dialysis (4D) study (1168 dialysis patients with type 2 diabetes mellitus) and the Ludwigshafen Risk and Cardiovascular Health (LURIC) study (2579 patients with coronary angiograms). Gal-3 level gradually increased with the severity of renal function: from 12.8 ± 4.0 ng/mL (eGFR ≥ 90 mL/min per 1.73 m2) to 54.1 ± 19.6 ng/mL (dialysis patients of the 4D study) [82].
Gal-3 plasmatic value after cardiac surgery was studied by Von Ballmos et al. for AKI prediction in 1498 patients and the highest tercile of Gal-3 was associated with severe AKI (OR of 2.95; p < 0.001) [83].
In a long term follow up study of 1320 patients with type 2 diabetes and an eGFR ≥ 30 mL.min−1 1.73 m−2, Tan et al. demonstrated that Gal-3 was independently associated with doubling of serum creatinine (HR 1.19 CI95%[1.14, 1.24], p < 0.001) even after adjusting for chronic renal risk factors, baseline eGFR, and albuminuria status [84].
3.2. Proteinuria
Kikuchi et al. reported a correlation between glomerular infiltrated Gal-3 positive monocytes and proteinuria in renal biopsies of 37 patients with glomerulonephritis (GN) (r = 0.616, p < 0.001) [86]. Moreover, in lupus GN, Kang et al. found a pronounced glomerular expression of Gal-3 in 81.8% (72/88) of patients associated with renal inflammatory cells. This overexpression of Gal-3 was correlated with histologic activity indexes, anti-dsDNA titers, and complement 3 and 4 levels [87]. In children, Ostalska-Nowicka et al. explored various types of GNs (minimal change disease (MCD), mesangial proliferation (DMP) and focal segmental glomerulosclerosis (FSGS)) and identified cortical and medullary Gal-3 positive cells highly expressed for no responding to steroid therapy (p < 0.001) [88].
Seventy-five patients with Mediterranean fever (FMF) with GN had higher serum Gal-3 levels compared to the control group and more importantly, for patients with proteinuria with a correlation ratio for proteinuria/creatinine of 0.785, p < 0.001. Prediction performance of serum Gal-3 for proteinuria had an AUC of 0.88 [89].
In a cross-sectional study including 90 patients, Gal-3 plasma level was significantly higher in macroalbuminuria (p ≤ 0.05) and for patients with poor kidney function (Stage IV–V CKD), with a prediction performance of 0.776 (CI95%[0.677, 0.875]; p ≤ 0.0001) [90].
Based on these studies, increased renal and plasmatic Gal-3 expression is associated with immune cells infiltration expressing Gal-3 in patients with glomerular injury.
3.3. CKD and Renal Prognosis
Alam et al. identified high plasma levels of Gal-3 in patients with severe comorbidities (heart failure, CKD) in 2 longitudinal cohorts, including patients with CKD: the Clinical Phenotyping and Resource Biobank (C-PROBE) study and the Seattle Kidney Study (SKS), which were associated (HR = 1.35, CI95%[1.01–1.80]) with chronic renal disease [91]. In a prospectively analyzed study from Atherosclerosis Risk in Communities (ARIC), Rebholtz et al. measured Gal-3 plasma levels in 9148 patients with no chronic kidney disease and no chronic heart failure. The authors showed that Gal-3 was higher for low estimated glomerular filtration rate, low urine albumin-to-creatinine ratio, and was associated with CKD with an OR of (2.22 CI95%[1.89, 2.60]) [92].
In another cohort including patients with chronic kidney disease, Gal-3 plasma levels were associated with elevated serum creatinine, urine protein/creatinine ratio, and were independently associated with CKD progression [93].
It was demonstrate the association and potential role of Gal-3 for poor renal prognosis and evolution of acute injury to chronic renal damage.
3.4. Transplantation
There is a major need for a good biomarker in kidney transplantation as it can allow therapeutic strategy changes. Sotomayor et al. analyzed 561 patients and baseline median Gal-3 of 21.1 (IQR [Q1:17.0, Q2:27.2] ng/mL. In this study, Gal-3 was associated with increased risk of graft failure (hazard ratios (HR) per 1 SD change, 2.12; CI95%[1.63, 2.75]; p < 0.001), more importantly for patients with hypertension (HR, 2.29; CI95%[1.80, 2.92]; p < 0.001) or smoking history (HR, 2.56; CI95%[1.95, 3.37]; p < 0.001) [95]
3.5. Mortality and Poor Cardiovascular Outcome
Finally, patients with renal diseases often have higher mortality rates. In 4D and LURIC, Gal-3 plasma high levels were significantly associated with all-cause mortality, or cardiovascular mortality, in a population with renal impairment compared to patients with no renal disease [82]. Hogas et al. confirmed in patients with hemodialysis and observed that a level of Gal-3 > 23.73 ng/mL was an independent predictor of mortality (HR: 2.60; CI95%[1.09, 6.18]) [96].
Later studies, in a big meta-analysis enrolling 5226 patients, Zhang et al. confirmed this association between Gal-3 and an increased risk of all-cause mortality and cardiovascular (CV) event in CKD patients (HR:1.379, [1.090, 1.744]) and associated with the risk of CV events in CKD patients (HR = 1.054, CI95%[1.007, 1.102])[97].
Thus, Gal-3 has been demonstrated to be a good biomarker for poor cardiovascular prognosis, and more precisely in patients with AKI [98]. These results highlight the role of Gal-3 in cardio-renal syndrome as it is associated with poor renal and cardiovascular prognosis. Some preclinical studies have started to identify Gal-3 as a key player in the type 3 cardio-renal syndrome [61]. The mechanism in this context remains complex but associates both immune and tissue expression of Gal-3.
4. Gal-3 as a Therapeutic Target and Perspective
Lau et al. evaluated the use of MCP in hypertensive cardiac complications in a randomized controlled trial and found that inhibition of Gal-3 did not influence fibrosis cardiac biomarkers expression, but was slightly associated to a diminution of plasmatic creatinine and an increase of eGFR in MCP-treated patients. This suggests a potential interest of using Gal-3 inhibitors in patients with renal injury but data remain insufficient to draw definitive conclusions [101].
Gal-3 has also been evaluated as a treatment of fibrosis in pulmonary disease. Hirani et al. treated 36 healthy patients and 24 patients with pulmonary fibrosis with inhaled Gal-3 inhibitor and reported a good tolerance in healthy patients and a diminution of plasmatic markers associated with pulmonary fibrosis [102]. Some studies used Gal-3 inhibitors in the treatment of cancer, using drug resistance and survival endpoint [103], but they remain insufficient for therapeutic recommendations. Nevertheless, clinical data are missing to identify the impact of inhibition in clinical care and particularly for improving renal outcome. Preclinical data are promising, but the pathophysiology of renal protection remains unclear. Additional studies are needed to propose this treatment in a clinical point of view.
A deeper investigation of Gal-3 or related pathways has multiple perspectives. First, the use of Gal-3 as a biomarker is a major breakthrough for the clinical management of patients with renal disease and can help to monitor and guide specific therapeutic management. Second, even though its specific therapeutic use has not been established yet, Gal-3 can help stratify patients with renal damage. More preclinical studies are needed to confirm Gal-3 inhibition as a potential therapeutic target to limit renal injury.
This entry is adapted from the peer-reviewed paper 10.3390/ijms23063124
This entry is offline, you can click here to edit this entry!
