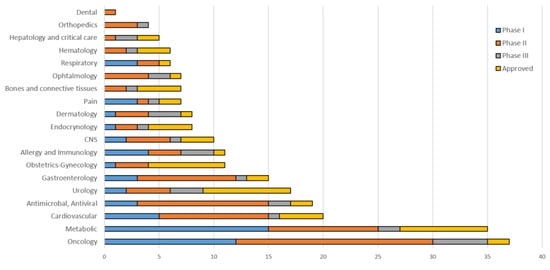
5. The Main Strategies for Developing the Next Generation of Peptide Drugs
The approaches used to increase peptide stability are continuously being improved, leading to new kinds of structural modifications [
33,
34]. One apparent solution for stabilizing the hydrolytic lability of drugs containing natural peptides is to incorporate modified analogs of natural peptides previously registered as parenteral drugs. Analog modifications are based on introducing substitutions in various parts of the original molecule in order to stabilize and sometimes change its structure, spectrum, and even direction of action [
35].
An essential requirement for improving peptide structure is an ability to minimize the possible toxicity of the obtained analogs. Currently, many laboratories [
36] are developing peptide-modification strategies to increase the binding affinity to receptors or active centers of enzymes, as well as their absorption, distribution, metabolism, and excretion profile (known as the “ADME” profile) [
37].
Novel synthetic strategies allow for modulating pharmacokinetic properties and target specificity through amino acid or backbone modification by incorporating non-natural amino acids and conjugating moieties that extend half-life or improve solubility. Substituting natural amino acids is one strategy used to prevent hydrolysis, where modifications are introduced at sites that undergo hydrolysis, followed by replacing the original amino acid [
3]. The substituents can be
d-amino acids, β-amino acids, dehydroamino acids, and various olefin derivatives. Such modifications improve the stability and increase the half-life of the peptide molecules in plasma [
11,
38]. Various critical issues associated with therapeutic peptide delivery have drawn increasing attention to the development of new formulations for alternative routes of administration, such as oral, nasal, buccal, pulmonary, transdermal, rectal, and ocular [
39]. Penetration of drugs through oral mucosa into the systemic circulation is a major hindrance in their absorption, as the oral route easily degrades a hydrophilic, large-molecular-weight drug (e.g., proteins and peptides), resulting in their decreased availability in systemic circulation [
40].
Examples of modifications include the introduction of proline and hydroxyproline (both resistant to protease degradation) into cleavage sites to replace easily hydrolyzed amino acids in order to improve in vivo drug stability [
41,
42]. In addition, N-methylation or the introduction of N-methyl-amino acids has also been used to increase peptide stability, reduce possible hydrogen bonding, and improve permeability [
43]. Moreover, the simultaneous inclusion of
d-amino acids and N-methylation at amide bonds can significantly increase metabolic stability, thereby creating additional steric hindrance. Furthermore, many structural modifications, including N-alkylation, can increase the biological and metabolic stability of peptides [
44,
45].
Proteolytic enzymes in the blood, plasma, liver, or kidney include exopeptidases, aminopeptidases, and carboxypeptidases, which hydrolyze peptide sequences from N- and C-termini. Therefore, N-acylation and C-amidation can potentially increase the resistance of modified peptides to proteolysis [
46]. Linear cyclization is a generally accepted method of increasing protein rigidity, with this process resulting in the formation of intramolecular hydrogen bonds and decreasing intermolecular hydration. Head-to-tail peptide cyclization offers the advantage of strengthening the peptide chain, stabilizing the conformation, and inhibiting cleavage by endopeptidases. Therefore, cyclization might represent the simplest method to prolong the half-life of a peptide in vivo, as it often increases the biological activity of a peptide [
35]. Moreover, introducing N-terminal
d-amino acids can suppress degradation by exopeptidases, similar to reducing C-terminal carboxyl groups into a corresponding alcohol moiety [
47].
The chemical “stapling” of amino acid side chains onto a peptide chain can be achieved via the insertion of residues into a peptide chain through hydrocarbon “inserts” or by forming lactam bridges to stabilize peptide helicity and increase their stability and intracellular permeability. The so-called “stapled-peptides” method is gaining popularity [
48,
49]. Another modern approach to increasing peptide stability and creating a more durable compound is to conjugate peptides with macromolecules. Various polymers have been applied for these purposes, including polyethylene glycol (PEG) [
50] and polyvinylpyrrolidone, as well as the use of protein carriers, such as albumin. PEGylating peptides can effectively reduce their potential immunogenicity, maintain their biological activity, and slow down enzymatic hydrolysis [
51]. In addition, some fatty acids are used to stabilize peptides and protect them against proteolysis. Peptide molecules are encapsulated into liposomes, nano/microparticles, or micelles with a higher molecular weight [
52] to increase the half-lives and bioavailabilities of peptide drugs [
53].
Conjugating peptides with lipids confer lipopeptide derivatives with new structural and biological properties that result in compounds with improved potency and selectivity. Lipidation of peptides leads to the formation of amphiphilic peptide conjugates with increased bioavailabilities and increased capability to cross cell membranes [
54]. Recently, a new concept for creating full-length enantiomeric
d-peptides, which involves replacing all
l-amino acids with the corresponding
d-amino acids, has become widespread, with such peptides (
d-peptides) showing significantly improved stabilities and half-lives [
55,
56].
One of the first natural peptides to be successfully modified was the hormone vasopressin, which contains
l-Arg and has a half-life in humans of 10 to 35 min [
57]. Vasopressin analogs containing
d-Arg instead of
l-Arg are called desmopressin and have a half-life of ~4 h [
58]. An analog of somatostatin (the drug octreotide, which is used to treat gastrointestinal tumors) has a shorter sequence than somatostatin (8 amino acids instead of 14) and
l-amino acid substitutions for the corresponding
d-amino acids[
59].
The minimal cyclic structures of peptide compounds are 2,5-diketopiperazines (DKPs), which are cyclodipeptides obtained by condensing two α-amino acids.
Numerous different structures can be generated based on DKP in order to search for new lead compounds [
60]. DKP derivatives are often found in nature both in the form of simple unsubstituted 2,5-DKP structures and more complex molecular structures in natural products, fungi, bacteria, plants, and mammals. For example, many antibiotics are DKP derivatives [
61]. Drugs have been developed with structures ranging from simple cyclic dipeptides, such as derivatives of cycloserine dimers [
62] or kairomycin B [
63], to complex conjugated polynuclear systems, such as bicyclomycin [
64].
2,5-DKP is resistant to proteolysis and an attractive target for structural and functional studies aimed at searching for new potential drugs. These conformationally limited chiral centroids have six positions available for structural modification by various functional groups with specific stereochemistry. The 2,5-DKP structure enables alterations at all six positions and stereochemical isomerization at all four positions of the optical centers. In addition, 2,5-DKP has a rigid framework that can mimic the preferred conformation by limiting the mobility of amino acids embedded in its structure. The 2,5-DKP structure comprises trifunctional amino acids containing various functional groups, which can be used to identify target positions with which this molecule interacts and to serve as linkers for attaching multiple functional groups (pharmacophores).
We previously developed an original platform for modifying peptides by synthesizing peptidomimetics based on substituted 2,5-DKP [
65,
66,
67]. Pharmacophores should be able to easily undergo metabolic transformations, such as ester bond formation with the centroid and easy hydrolysis in the body. 2,5-DKPs are relatively easy to synthesize and can accommodate a wide variety of substituents (i.e., various amino acids used as building blocks). The large set of substituents makes it possible to widely vary the physicochemical characteristics of the molecule, including its structure, size, shape, lipophilicity, dipole moment, electrostatic charge, and functional groups. This flexibility enables in silico modeling of analogs for directed library design [
68].
When looking for new lead compounds, it is critical to not introduce changes in the centroid or substitutions in the attached groups that can lead to toxicity. One reason explaining the different physiological activities of drug stereoisomers is the differences in their penetration into an organism, which may be due to the structural features of 2,5-DKP, the properties of biological membranes (which are produced from optically active, asymmetric material), and the presence of transport systems that transport metabolites across membranes [
69]. In one approach that utilizes 2,5-DKPs [
70] (both short peptide analogs and versions containing “inserts” at different positions in the chain), the DKP moiety is positioned at the N- or C-terminal end of the molecule or within the peptide. The use of this approach has become widespread and can increase the hydrolytic stability and possibility of oral administration [
71]. Furthermore, some derivatives of branched DKPs can exhibit hemostimulatory [
65] and immunosuppressive properties [
66], with one study demonstrating an acquisition of several new drugs based on 2,5-DKPs [
68].