Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Medicine, General & Internal
Studies found that all immunizations were safe, with very few or no Side Effects (SEs); however, the form of SEs was shown to be more persistent in DNA- and mRNA-based vaccines, whereas inactivated viral vaccines were associated with longer-duration SEs. Overall, SEs were shown to be more prevalent in women and youngsters. Certain instances of adverse responses have also been observed, although their pathological relationship with COVID-19 immunization has yet to be determined.
- COVID-19 vaccination side effects
- AstraZeneca side effects
- Pfizer side effects
- Moderna side effects
- Sinopharm side effects
- Sputnik V side effects
1. Side Effects (SEs) and COVID-19 Vaccination Resistance
The development of a COVID-19 vaccine was viewed as a critical strategy for ending the pandemic. Public acceptance, on the other hand, is based on people’s ideas and perceptions of the vaccination [13]. The rates of vaccine hesitation and rejection are still higher, which may require legislative changes to make the vaccination program profitable [12]. One possible cause behind neglecting immunization could be the negative emotions such as anxiety, depression, anger, and irritability which have already been observed in many studies during quarantine periods. This occurs because psychosocial disturbances, such as relational loss and social rejection, cause changes in mind–body interplay [14]. Such hesitation has been linked to more negative beliefs that the vaccination would cause SEs or be unsafe [15]. The COVID-19 vaccine’s adverse effects play a critical role in public trust in the vaccination and its administration technique [3]. An online self-administered questionnaire, completed in May 2020 among the Saudi population regarding vaccination views and the potential barriers preventing people from becoming vaccinated against COVID-19, cited SEs as major obstacles to vaccine uptake [13]. Similarly, in another study, seven attributes were evaluated to create vaccine choice sets: vaccine effectiveness, SEs, accessibility, number of doses, vaccination sites, length of vaccine protection, and a fraction of acquaintances vaccinated. Although all seven factors were found to have a substantial impact on respondents’ vaccination decisions, and vaccination SEs were among the most relevant factors [16,17], the likelihood of a serious adverse SE was found to be a modest but significant cause of vaccination rejection. In comparison to rates of 1/1,000,000 general SEs, a significant adverse SE rate of 1/100,000 was more likely to discourage vaccine usage [18]. COVID-19 vaccinations have been expedited through the review process due to the lack of safety data. COVID-19 vaccines had a low level of public acceptance of 37.4%. Therefore public health officials must implement systematic interventions to reduce vaccine hesitancy and improve vaccine acceptance [19] and more studies are required to identify the benefits and SEs.
2. Studies Reporting SEs of COVID-19 Immunizations
Previously, vaccination safety research only came from manufacturer-sponsored studies, but many other cross-sectional survey-based studies around the world have helped in the generation of vaccine-related safety data reports [3]. COVID-19 vaccine types and their SEs are illustrated in Figure 1.
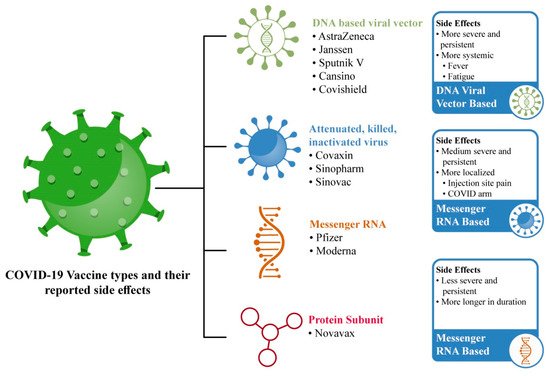
Figure 1. Most-utilized COVID-19 vaccinations and their frequently reported SEs.
2.1. Messenger RNA Based COVID-19 Immunization
The FDA granted Emergency Use Authorization (EUA) to two two-dose mRNA vaccines: BNT162b2 from Pfizer–BioNTech, for people aged ≥16 years; and mRNA-1273 Moderna for people aged ≥18 years [32]. Both vaccines employ either lipid nanoparticle delivery technology or a modified mRNA-delivery mechanism. Modified mRNA is used to encode the COVID-19 spike proteins, with mutant mRNA being added to lock them into the three-dimensional structure required to cause an interaction between the spike proteins and virus-neutralizing antibodies. They have a higher safety profile than other viral vaccines since they are not created with actual infections and are not incorporated into host DNA [28]. Both have been considered safe during pregnancy [29]. Messenger RNA vaccinations generate milder, less-frequent systemic adverse effects, but more localized SEs [9,33].
2.1.1. Pfizer–BioNTech (BNT162b2) COVID-19 Vaccination
Millions of people worldwide have been immunized with the Pfizer–BioNTech vaccination [24]. The Pfizer–BioNTech (BNT162b2) vaccine has shown good safety and efficacy in phase 3 trials and reduces the chances of SARS-CoV-2 infection after approximately 12 days of vaccination [34]. The Pfizer–BioNTech vaccine was associated with considerably greater rates of all forms of adverse reactions [35]. Among these eleven studies on Pfizer, eight studies reported headache; seven studies reported weakness/fatigue and myalgia/muscle/body pain; six studies reported local injection site/shoulder pain, chills/feeling cold, and fever; four studies reported enlarged lymph nodes and joint pain/arthralgia; three studies reported nausea/vomiting/GIT disturbances; one study reported cutaneous urticarial/morbilliform eruptions, weakness, hand numbness, mucosal lesions, taste disturbances, skin burning, rash, allergic reactions, dry cough, sore throat, brain fog, and decreased sleep quality. On the other hand, the prevalence of major or complex SEs has also been reported in these nine studies, including one study which reported adverse skin reactions, i.e., chilblains; zoster, herpes simplex, pityriasis rosea, etc.; two studies, which reported severe allergic responses such as anaphylaxis; one study which reported thromboembolic events such as a cerebrovascular accident, myocardial infarction, pulmonary embolism, acute hypertension (over 210/105mm Hg), and Bell’s palsy.
2.1.2. Moderna (mRNA-1273) COVID-19 Vaccination
The Moderna vaccine, similar to the Pfizer vaccine, received FDA approval in its early clinical efficacy trials [29,63]. The SEs recorded for this vaccination are reasonably similar to those reported for other vaccines [63]. The most reported SEs include injection site pain, headache, fatigue, muscle pain, malaise, chills, joint pain, mucosal lesions, oral paresthesia, taste disturbance, pruritus, rash, itchy sensations in the mouth and throat, sensations of throat closure, muscles spasms, anorexia, decreased sleep quality diarrhea, flushing, nasal stiffness, and respiratory symptoms. Local injection site reactions, such as urticarial eruptions and morbilliform eruptions, have also been reported.
2.2. Viral Vector-Based COVID-19 Immunization
The non-replicating adenoviruses used in viral vector-based vaccines are safe for humans. This vaccine’s technique has been in use for decades. Two distinct adenoviruses (AD16 and AD5) are employed, one in each dose of vaccine, with a 21-day interval [76]. The prevalence of systemic SE was higher in the AZD-1222 vaccine than in the Sputnik V and Covaxin vaccines [44].
2.2.1. AstraZeneca (ChAdOx1 nCoV-19) COVID-19 Vaccination
The AstraZeneca (ChAdOx1 nCoV-19) vaccine has shown good safety and efficacy in phase 3 trials and reduces the chances of SARS-CoV-2 infection after about 12 days of vaccination [34]. The AstraZeneca ChAdOx1 nCoV-19 (AZD1222) vaccine has been linked to thrombosis with thrombocytopenia syndrome (TTS) in 3/100,000 people, with high fatality rates reported in many countries. In Australia, the potential risks of the AZD1222 vaccine in younger adults who are unlikely to die from COVID-19 may outweigh the benefits [77].
2.2.2. Covishield (AZD1222)
Covishield is a COVID-19 vaccine developed in India. Covishield is currently in use and has nearly 90% effectiveness. It was developed by Oxford–AstraZeneca and is manufactured by the Serum Institute of India (SII) in Pune, Maharashtra. It employs the same technology that was used to develop vaccines for viruses such as Ebola, a chimp adenovirus, namely, ChAdOx1. This technology has been modified to carry the COVID-19 spike protein into human cells. A web-based survey study reported the SEs of the Covishield vaccine. The prominent SEs were mild fever (28.91%), myalgia (26.43%), cold and cough (8.16%), headache (6.74%), and local injection site pain (3.37%). Less-prevalent SEs included fatigue, diarrhea, rigors, joint pain, and nausea. Only 0.70% of recipients claimed severe symptoms with admission and observation in clinical setups [53].
2.2.3. Janssen (Ad26.COV2.S) COVID-19 Vaccination
The Janssen vaccine is based on the genetic modification of inactivated adenoviruses by the deletion of the E1 gene, which is replaced with the spike gene [78]. A VAERS-based study reported 64 anxiety-related events. Other SEs included tachycardia, hyperventilation, dyspnea, chest pain, paresthesia, light-headedness, hypotension, headache, pallor or syncope, and fainting. Three clinical cases of severe immune thrombocytopenia (ITP), severe cutaneous adverse reaction, and Guillain-Barré syndrome (GBS) have been reported in its major complications.
2.2.4. Sputnik V (Gam-COVID-Vac) COVID-19 Vaccination
Russia’s first authorized vaccination was developed and manufactured wholly in the country, and its name alludes to the 1950s space race. To create the vaccine, the adenoviruses are mixed with the SARS-CoV-2 spike protein, which causes the body to respond with an immunological response [79]. Sputnik V was registered as Gam-COVID-Vac by the Russian Ministry of Health in August 2020, and it has been delivered in 61 countries globally since December 2020 [48].
2.3. Inactivated COVID-19 Immunization
2.3.1. Covaxin Vaccine (BBV152)
India has produced a COVID-19 vaccine—namely, COVAXIN—that is an inactivated vaccine produced by Whole-Virion Inactivated Vero Cell-derived platform technology. It does not require reconstitution or sub-zero storage and comes in ready-to-use multi-dose vials that are stable between 2 and 8 °C. It cleared Phase I and II human clinical trials in July 2020. Vaccine-induced antibodies, according to the National Institute of Virology, can neutralize the UK variant strains as well as other heterologous strains. The effectiveness of the Covaxin vaccine is nearly 81% [53]. Local injection site pain, fatigue, muscular pain, and fever were described in a single investigation on the SEs of a Covaxin dose [44].
2.3.2. Sinovac
The most common local SE 54.6% was pain, while the most common systemic SEs were fatigue 39.2% and headache 34.1%. Two-thirds of individuals who were vaccinated reported at least one local or systemic SE, with women and people under the age of 35 being the most affected. Individuals who worked more than 8 h a day felt the vaccine’s local adverse effects, such as increased hunger and weariness, more acutely [54].
2.3.3. Sinopharm (BBIBP-CorV/Vero Cells) COVID-19 Vaccination
The Sinopharm vaccine is a complete viral inactivated vaccine manufactured from Vero cells. These cells replicate the SARS-CoV-2 virus, which is subsequently treated with beta-propiolactone, which deactivates the virus by binding to its genes [28]. Sinopharm post-vaccination SEs remain modest and predictable for the first and second doses, with no cases of hospitalization, which help to reduce vaccine hesitancy [51,80]. Sinopharm vaccine recipients had a longer duration of adverse effects. The majority of these adverse effects are minor and curable [35].
3. COVID-19 Vaccinations’ Reported Minor Side Effects
The most-prevalent minor SE reported after COVID-19 vaccinations were localized reactions in the form of local injection site reactions, injection site/shoulder pain swelling, and soreness surrounding the area, whereas the most-prevalent generalized SEs were headache, fever, sweating, chills, tiredness, and fatigue. There were minor SEs in the nose, throat, and oral cavity, including throat infection or irritations, breath tightness, nasal stuffiness, flu-like symptoms, sensations of throat closure, oral mucosal lesions, paresthesia, and taste disturbance. The minor SEs of musculoskeletal symptoms included joint pain, muscular spasm, whole-body aches/myalgia, osteoarticular pain, back pain, and neck pain. The SEs of skin included skin rashes or allergic responses of urticarial eruptions, morbilliform eruptions, and pruritus. The minor SEs of the gastrointestinal system included nausea, vomiting, and diarrhea, while other SEs reported include fast heartbeat, dizziness, flushing, palpitations, brain fogging, mental confusion, anorexia, decreased sleep quality, drowsiness, hand numbness, enlarged lymph nodes, etc.
4. COVID-19 Vaccinations’ Reported Major Side Effects
The adverse or major-severity SEs reported from COVID-19 vaccinations on skin included zoster or herpes simplex flares such as varicella-zoster virus reactivation, chilblains, cosmetic filler reactions, and pityriasis rosea. The major SEs of the cardiovascular system (CVS) reported in studies include thromboembolic events such as thrombosis, cerebrovascular accidents, myocardial infarction, takotsubo cardiomyopathy, and pulmonary embolism. The major SEs of the central nervous system (CNS) included CNS demyelination, multiple sclerosis, syncope, transverse myelitis, encephalopathy, stroke, and acute disseminated encephalomyelitis. The major SEs of musculoskeletal system included Guillain-Barré syndrome, Bell’s palsy and facial palsy. Ocular adverse effects included episcleritis, anterior scleritis, acute macular neuroretinopathy, acute middle maculopathy, subretinal fluid, and anterior uveitis associated with juvenile idiopathic arthritis (JIA). Severe immune system disturbances and autoimmune side effects were also observed, such as antineutrophil cytoplasmic autoantibody (ANCA)-associated vasculitis of acute kidney injury, severe immune thrombocytopenia, lympho-proliferative disease, hypersensitivity reaction, and severe cutaneous adverse reaction with panhypopituitarism secondary to craniopharyngioma resection. Furthermore, among adverse reactions, COVID arm with pruritic, erythematous plaques were reported. However, the link between the mildness and severity of SEs, along with the mechanism involved and other risk factor associations, is still under debate and requires proper investigative studies.
This entry is adapted from the peer-reviewed paper 10.3390/vaccines10040488
This entry is offline, you can click here to edit this entry!
