3. Intracellular Effects of Phytocannabinoids in Glioma Cells
Considering the complexity and the wide distribution of the endocannabinoid system (ECS) components and their interaction with phytocannabinoids [
47,
49], phytocannabinoids may have the potential to impact and mediate a multitude of cancer-related signaling pathways. One common pathway activated by phytocannabinoids in different cancer types is the ER-stress pathway, which is one of the main mechanisms to induce apoptosis of glioma, astrocytoma, melanoma, and pancreatic tumor cells [
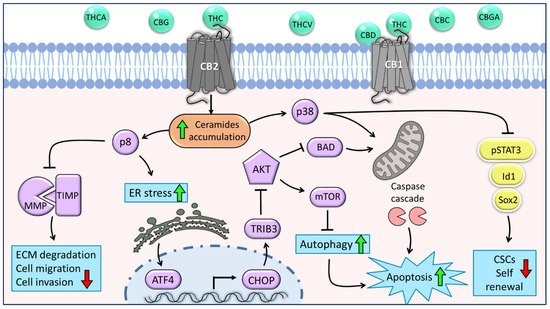
56]. Previous studies on several models of glioma reported that CB1 receptor agonists and, more efficiently, CB2 receptor agonists stimulated the synthesis and accumulation of ceramide, a pro-apoptotic lipid second messenger which leads to the induction of stress protein p8 ([
31,
57];
Figure 1). Following this p8 induction, downstream ER-stress-related genes were induced (
Figure 1), and as a result, the intrinsic mitochondrial pathway was activated [
31,
57].
Figure 1. The main molecular mechanisms underlying the anti-tumor effects of C. sativa phytocannabinoids on glioma cells and glioblastoma stem cells. Phytocannabinoids inhibit cell viability and motility through various cannabinoid receptor (CB)-mediated mechanisms. THC acts as an agonist of both CB1 and CB2 receptors; CBD may act as a CB1 antagonist. The activation of CB1 or CB2 stimulates the synthesis and accumulation of ceramides (orange shape) and, as a result, triggers the induction of p8. This leads to the inhibition of cell migration and invasion through the downregulation of MMPs. Furthermore, p8 promotes the upregulation of ER-stress-related genes ATF-4, CHOP, and TRIB-3, followed by inhibition of the Akt-mTORC1 axis and initiation of autophagy, which is upstream of apoptosis. In addition, inhibition of Akt leads to the overexpression of BAD and consequently induces apoptosis via the intrinsic mitochondrial pathway. Another signaling pathway activated by ceramides is p38-MAPK, which involves both apoptosis activation and inhibition of CSC self-renewal through the downregulation of stemness regulators, such as p-STAT3, Id1, and Sox2 (yellow shapes). Green arrows represent upregulation and red arrows represent downregulation of biological processes. Purple shapes represent genes or proteins, and blue shapes represent biological processes.
Recently, we have shown that CBG-rich and THC-rich combinations of phytocannabinoids induced Activating transcription factor 4 (
ATF4), C/EBP homologous protein (
CHOP)-
10 (GADD153/DDIT-3), and Tribbles homolog 3 (
TRIB3) gene transcription in a CB2 activation-dependent manner ([
37];
Figure 1), supporting the notion that phytocannabinoid treatments induce cell death via ER stress. ATF4 is a transcription factor transiently induced following treatment with ER stressors [
56]. In turn, ATF4 induces
CHOP expression, a transcription factor that regulates the expression of many pro- and anti-apoptotic genes [
58]. Under ER-stress, CHOP activates pro-apoptotic proteins, including the B cell lymphoma-2 (BCL-2) family proteins, such as BAK and BAX, and represses anti-apoptotic BCL-2 family proteins [
58]. TRIB3 is a pseudokinase and another protein associated with ER-stress, which was found to facilitate ER-stress-dependent apoptosis via the NF-κB pathway [
59]. Moreover, TRIB3 has been shown to inhibit the Akt-mTORC1 axis, consequently leading to the initiation of autophagy (
Figure 1), which is upstream of intrinsic mitochondrial apoptosis [
60].
Furthermore, treatment with the cannabinoid-receptor synthetic agonist WIN-55,212-2 led to upregulation of the BCL-2 homology 3 (BH3)-only family member BAD, a pro-apoptotic protein, in response to ceramide activation and the serine/threonine kinase Akt downregulation in glioma cells ([
61];
Figure 1). Ceramide is also an important regulator of p38 mitogen-activated protein kinase (MAPK), and previous studies on human leukemia and glioma cells reported that following THC treatment, activation of this pathway induced apoptosis partially via the CB1 and CB2 receptors ([
57,
62];
Figure 1).
Importantly, in contrast to malignant cells, normal brain cells, such as primary neurons and astrocytes, do not undergo apoptosis or present ceramide accumulation in response to phytocannabinoid treatments [
31]. In addition, it has been shown in vivo that even at high doses, there is no sign of any damage or neurotoxicity to normal brain tissue following treatments with phytocannabinoids [
63]. These findings, together with the differences in the expression of cannabinoid receptors between normal tissues and cancer cells, and the fact that cannabinoid receptors mediate the anti-cancer activities support the suggestion that cannabinoid receptors regulate cell survival and cell death signaling pathways differently in glioma cells and non-transformed cell [
64].
Although the role of cannabis compounds in the suppression of cancer migration and invasion is elusive and poorly characterized, accumulating evidence suggests that cannabis compounds have potent anti-migrative and anti-invasive effects on GBM cells, both in vitro and in vivo. It was previously reported that treatment with THC or CBD down-regulated the expression of major proteins associated with glioma tumor migration, in particular MMP-2, MMP-9, TIMP-4, and TIMP-1 [
34,
65], even at low concentrations, which were insufficient to induce cell apoptosis. TIMP-1 and some MMP expression is selectively upregulated in different cancers and strictly associated with tumor malignancy and metastasis [
66]. Interestingly, THC treatment depressed TIMP-1 and MMP-2 expression in glioma cell lines as well as in cultured human GBM primary cells. In addition, the local administration of THC down-regulated TIMP-1 and MMP-2 expression in glioma-bearing mice and in two patients with recurrent GBM [
30,
65]. Moreover, these effects of THC were suggested to be mediated via CB2 receptor activation and were prevented by the blockade of ceramide synthesis and by knock-down of the p8 stress protein in glioma cells ([
65];
Figure 1).