Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Among nutrients to cope with aging in special cognitive decline, the long-chain omega-3 polyunsaturated fatty acids (ω-3 LCPUFAs) docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA), have emerged as very promising ones. Due to their neuroinflammatory resolving effects, an increased status of DHA and EPA in the elderly has been linked to better cognitive function and a lower risk of dementia. Recently, supplementation with structured forms of EPA and DHA, which can be derived natural forms or targeted structures, have proven enhanced bioavailability and powerful benefits.
- structured lipids
- omega-3 PUFAs
- cognitive function
- cell senescence
- DHA
- EPA
- omic technologies
1. Introduction
After the last hundred years of medical and life science technology progress and advances, people now live longer than ever before. Currently both preventive and therapeutic approaches are failing to reduce non-communicable diseases (NCDs), but they have succeeded in increasing our life-expectancy. Consequently, although human lifespan has significantly increased, our healthspan has not kept up the pace [1].
Demographically, society is getting older and the aging process includes progressive and irreversible biological changes, resulting in a growing risk of suffering from chronic diseases, cognitive impairments, physical disfunctions, and an increased probability of dying [1]. In fact, the loss of cognitive function is considered the most critical change during aging and it is projected that the patients with dementia—considered as significant loss of cognitive function which should be distinguished from neurodevelopmental disorders, such as intellectual disability [2]—will reach up to 115.4 million in 2050 [3].
The aging process is conditioned by the interactions between our genetic inheritance and environmental influences. While aging, our cells are submitted to a wide range of intrinsic and extrinsic insults, including oncogenic activation, oxidative and genotoxic stress, mitochondrial dysfunction, irradiation, and mutagenic agents [4]. In response to these disturbances, a stable state of cell cycle arrest happens and the cellular ability to proliferate decreases entering into a phase of senescence [4].
Senescent cells undergo morphology changes, chromatin remodelling, metabolic reprogramming and secrete a complex mix of mostly proinflammatory factors, like IL-1 and TNF-α. As senescent cells are more abundant, it leads to a potentially chronic inflammatory state independent from the activation of immune cells, which may impair tissue homeostasis [5]. This phenomenon of chronic low-grade systemic inflammation is called “inflammaging” and is considered to play a central role in the pace of aging, in the impairment of cognitive and physical functions and lastly, in the development of age-related disease.
In addition, many intrinsic and environmental factors generate oxidative stress which is a common phenomenon caused by an imbalance between production and detoxification of free radicals. Free radicals, mainly reactive oxygen and nitrogen species (RONS), can damage cells and tissues leading to the activation of proinflammatory pathways which contribute to the above mentioned “inflammaging” [6] leading to a higher degree of cellular senescence.
To improve the healthspan and the quality of life in the elderly, it is crucial to consider the role of preventive health interventions. Preventing disease, not only has positive health and well-being outcomes, which is the most important impact, but also wider economic significance, since the healthcare system is not prepared to handle the pressure of an aged society. Consequently, the goal of the scientific community is to find non-pharmacological therapies to prevent the most common age-related disfunctions and especially, those related with the loss of cognitive function, thus extending the well-being and optimal health of aging people for the longest possible time [7].
Following this approach, two powerful and recent strategies, functional foods and exercise, have been shown to decrease the risk of aging-related diseases. As nutrition is closely linked with health status, there is a growing demand for appropriate dietary patterns that include food supplements and functional foods to address healthy aging. Bioactive compounds with proven anti-inflammatory and antioxidant effects are suitable as anti-aging ingredients, but few of them like vitamin E, vitamin B12 and B6 or tea polyphenols have shown consistently improved cognitive effects [8][9][10][11].
Among nutrients assessed for brain health, the omega-3 polyunsaturated fatty acids (ω-3 PUFAs) must be highlighted, specifically the ω-3 LCPUFAs: DHA and EPA. Increased intake of ω-3 LCPUFAs, which are mainly found in fish and other seafood, have been associated with better cognitive function, slower rates of cognitive decline and an overall lower risk of developing dementia [12][13]. Furthermore, DHA and EPA are promising bioactive ingredients in the treatment of more severe neurological age-related disease like Alzheimer’s or Parkinson’s disease [14][15]. However, some clinical studies with healthy subjects have failed to prove a direct effect between cognitive function improvement and ω-3 LCPUFAs supplementation during aging [15].
Thanks to the research of bioactive compounds for healthy aging, new structured forms of ω-3 LCPUFAs have emerged as promising ingredients with more powerful effects than simpler forms of DHA and EPA. This group of structured PUFAs comprises a wide range of larger structures, like triglycerides, phospholipids or derived lipid mediators of ω-3 LCPUFAs, and they have been proven to have greater bioavailability and stronger anti-inflammatory and antioxidant effect than conventional ω-3 PUFAs [16][17] according to the findings from in vitro and preclinical studies.
2. Senescence, Aging and Bioactive Compounds
When the phenomenon of senescence starts to grow in the nervous system, brain function impairment occurs. To understand the relationship between senescence onset and its negative implications on the nervous system and brain function, and how nutrition can modulate this relationship, the biological mechanisms of senescence should be updated.
2.1. Nutrients and Anti-Cellular Senescence Targets
As stated above, senescence is a physiological stress response of mammalian cells that results in the development of senescent cells (SC) with distinct physical, molecular, and metabolic signatures. Based on the type of induction, three broad categories of cellular senescence are defined: replicative senescence (telomere attrition), oncogene-induced senescence (activation of oncogenes), and genotoxic or oxidative stress-induced senescence.
Regardless of the different triggers of cellular senescence, SC are invariably accompanied by impaired mitochondrial functions, increased intracellular RONS and production and activation of the DNA damage response. Late SC, exhibit characteristic secretion of a milieu of cytokines and growth factors causing a chronic inflammation state independent from the activation of immune cells which may impair tissue homeostasis, a phenomenon known as inflammaging as mentioned above [18]. As a strategy to fight against tissue homeostasis impairment, three nutrition-mediated anti-cellular senescence targets may be established, according to the current literature.
2.2. Omega-3 LCPUFAs to Cope with Senescence
Blood levels of EPA and DHA are in the low range for most of the world population [19] despite them being promising bioactive nutrients contributing to healthy aging. Both ω-3 LCPUFAs modify cellular function through overlapping and distinct mechanisms of action. An increased intake of EPA and DHA exerts an enhanced appearance of those fatty acids in the membrane phospholipids of cells [15][20].
3. Bioactive Compounds and Improvement in Cognitive Function
A progressive decline in memory, language, problem-solving and other cognitive skills that affects a person’s ability to perform everyday activities, leads to mild cognitive impairment and may progress to dementia [21].
As mentioned previously, late senescent cells and changes in microglial function (immunosenescence) cause neuroinflammation and oxidative stress due to an enhanced pro-inflammatory cytokine production and weak redox homeostasis. Inflammation and ROS drive a progressive impairment of brain cell processes, such as neural membrane fluidity reduction, less synaptic plasticity and low neurogenesis [22]. These impaired functions may lead to irreversible neural changes like loss of grey and white matter volume, and significant alterations in memory, learning abilities and spatial recognition which have been described in both humans and animals [22][23] driving the age-related cognitive decline.
Although there is much to be clarified about the specific molecular mechanisms in which dietary components influence cognitive function, a growing literature supports the idea that certain dietary patterns and some bioactive compounds are able to modulate brain structure and function, exerting their beneficial influence throughout the entire lifespan [10][24].
Vitamins of the B group have been studied for their potential effect on cognitive function because of their role in homocysteine metabolism, specially vitamins B6 (pyridoxine), B9 (folate) and B12 (cobalamin) [11]. Several clinical studies have found that raised concentrations of homocysteine in plasma might be associated with increased risk of dementia in people older than 65 years [25]. Supplementation, over 3 years, of 0.8 mg a day of folate—higher than twice the recommended daily intake [26]—improved cognition in participants aged 50–70 years, but the intervention was more effective in those with high baseline homocysteine concentrations [27]. In general, most clinical trials of B vitamins have found no association with cognitive function. Only individuals with high baseline homocysteine, low baseline vitamin B concentrations, or established cardiovascular and cerebrovascular disease may benefit most from vitamin B supplementation [10].
As the brain is highly susceptible to oxidative damage, it has been suggested that inadequate antioxidant defences might mediate the pathogenesis and progression of dementia [28]. Many antioxidant nutrients such as vitamin C, vitamin E [29], zinc [30] (cofactor for enzymes with antioxidative activity) and carotenoids [31], and non-nutrient food ingredients like polyphenols [32], anthocyanins [33], lignans [34] or allicin [35] have been proven to be beneficial for age-related cognitive impairment. But, as far as there is not a deficit of the mentioned nutrients or a pathological situation, clinical trials with supplementation of these mentioned antioxidant compounds have not demonstrated a beneficial effect on any cognitive outcome in healthy patients [10].
Despite this, among nutrients addressed for brain health, ω-3 PUFAs, specifically ω-3 LCPUFAs must be highlighted [9]. Aging is associated with decreased cerebral ω-3 LCPUFAs levels due to reduced absorption, lower ω-3 PUFA capacity to cross the blood-brain barrier [12], and decreased capacity to convert PUFAs into LCPUFAs in the brain. As a major neuronal membrane component, DHA regulates neurogenesis, synaptogenesis, and neural membrane fluidity, which in turn modulates the speed of cell signalling and neurotransmission. In comparison with DHA, EPA constitutes a minimal proportion of total brain LCPUFA, but EPA inhibits proinflammatory metabolism and promotes adequate cerebral blood flow [36]. Altogether, a large amount of evidence demonstrates that poor ω-3 LCPUFA status in brain and plasma is associated with age-related cognitive decline [37].
4. Omega-3 LCPUFAs against Age-Related Cognitive Impairment
Although DHA has a structural role as a major component neuronal membrane fatty acid, it is endogenously transformed in the nervous system to an endocannabinoid-like metabolite called N-Docosahexaenoylethanolamine (synaptamide). Mainly through the specific target receptor GPR110, a G-protein coupled receptor, synaptamide promotes neurogenesis, neurite outgrowth and synaptogenesis in developing neurons. GPR110 induces cAMP production and phosphorylation of protein kinase A (PKA) and the cAMP response element binding protein (CREB) [38]. This signalling pathway leads to the expression of neurogenic and synaptogenic genes and suppresses the expression of proinflammatory genes. GPR110 is highly expressed in the brain during development but also during adulthood, emphasizing its relevance in the hippocampal region where neurogenesis is still happening [39][40].
Once it was proven that the pharmacological inhibition of neuroinflammation improved memory in aged murine models [41], the anti-inflammatory role of ω-3 LCPUFA gained attention as a possible therapeutic nutrient for healthy aging. Recent studies with aged rats [42] and mice [22] from a preventive point of view, show an improvement in memory and cognitive skills after a reduction in microglial activation and neuroinflammation, following supplementation with EPA and/or DHA. Moreover, in animal models ω-3 LCPUFAs and SPMs also have proven neuroinflammatory resolving effects and cognition improvements in age-related diseases, like Parkinson’s [43][44].
In preclinical data, human trials show a negative correlation between increased dietary supply of ω-3 LCPUFAs and pro-inflammatory markers [45] but, a positive correlation with verbal performance and learning ability in elderly people with high risk of early cognitive decline [43]. In patients with coronary artery disease and potentially ischemic risk, which reduces cerebral blood flow and contributes to the development of dementia, a daily high-dose of 1.86 g of EPA and 1.5 g of DHA over a 30 months period enhanced cognitive function significantly in comparison with control [46]. Also in middle-aged to older adults with obesity, which accelerates cognitive decline by endothelial dysfunction (impaired vasodilatation) in the peripheral and cerebral vasculature, greater processing speed mediated by improvements in circulatory function was observed following fish-oil supplementation (total dose of 0.4 g EPA and 2 g DHA) [47]. Furthermore, a study with old adults, with no risk factors, who had self-perceived cognitive function impairment (bad memory, learning difficulty,…), reported lower levels of cognitive inefficiency in activities of everyday life following 24 weeks treatment with fish oil (daily dose of 1.6 g EPA and 0.8 g DHA) [48].
However, when the effect of ω-3 LCPUFAs are assayed in healthy older adults the results are controversial [36]. Despite reported meta-analyses highlighting the potential of ω-3 PUFA to improve memory [13] and cognitive decline [37], some interventional studies have changed the perspective of the actual benefits of ω-3 LCPUFA [49][50]. As an example, Van de Rest et al. did not find significant changes in any of the cognitive domains for either low-dose (0.4 g/day EPA-DHA) or high-dose (1.8 g/day EPA-DHA) fish oil supplementation groups compared with placebo in a cohort of healthy individuals aged 65 years or older [51]. More recently, Baleztena et al. reported that daily supplementation with 0.75 g of DHA and 0.12 g of EPA did not show an improvement in the global cognitive function in adults over 75 years of age. They just found an apparent improvement in memory loss when the study subjects were well nourished [52].
According to the literature, two main issues justify the divergences [15]. Firstly, the effect of DHA and EPA depends on the stage of cognitive health assessed. This is supported by Canhada et al. in a recent systematic review where they conclude that the most beneficial effect of EPA and DHA supplementation in Alzheimer’s patients can only be expected in the early stage of the disease [53]. Secondly, there were several weaknesses in the trial protocols designed to assess the effect of EPA and DHA, such as the variability of doses of DHA and EPA, the type of placebo used, the combination of treatments [54], the duration of treatment, the sample size, the ω-3 LCPUFA status of the participants and the cognitive outcomes/tests measured as primary and secondary variables [15].
Therefore, since clinical trials are performed with a wide range of target population and low consistency of biomarkers measured in the nutritional interventions, the results from clinical studies with ω-3 LCPUFA do not allow a consensus about how traditional forms of ω-3 LCPUFAs affect cognitive function and aging [55].
5. Structured Lipids: Innovative Omega-3 LCPUFAs Molecules for Healthy Aging
Structured lipids can be defined as chemically or enzymatically modified lipids that change the fatty acid composition and/or the positional distribution [56]. They are created to be applied in functional food and clinical nutrition because of their characteristics or bioactive properties [57][58]. These structures can be a replica of natural forms of lipids with special structures designed to have a specific function. Most of the studies mentioned above use ethyl ester (EE) forms of DHA and EPA, or oils rich in DHA and EPA-EE for supplementation. However, in natural matrixes containing DHA and EPA, like in blue fish or breastmilk, they can be found in more complex structures as phospholipids (PL) or triglycerides (TG), or in derived forms, like SPMs, synaptamide and precursors.
Studies comparing the effect of supplementation with structured TGs and PLs ω-3 LCPUFAs against EE have proven enhanced bioavailability and powerful benefits of the structured forms in comparison with EE [59][60][61]. Similar intestinal absorption capacity and bioavailability between TG and PL have been described in studies analysing the responses of blood biomarkers [62][63], however, a greater brain absorption has been observed when DHA is esterified to PL [64]. In contrast, supplemented diets with a source of PL-DHA, TG-DHA or a mixture of both, resulted in similar increases in brain DHA compared to a low ω-3 PUFA diet [65].
Positional distribution is also important in structured lipids. For example, depending on the position of the fatty acid in the glycerol backbone of the TG (three positions, sn-1, sn-2 and sn-3), higher or lower absorption of the fatty acids can be observed. After intake, hydrolysis of TGs is performed by lipases, which are typically enantioselective. In the digestive tract, fatty acids in the sn-1 and sn-3 positions of the glycerol backbone are cleaved while the fatty acids of the sn-2 position are mainly absorbed [56] as represented in Figure 1. In this sense, preclinical results show differences in bioavailability when dietary TGs had DHA in the sn-1, sn-2 or sn-3 position, explained by less secretion of fecal DHA when this was at the sn-2 position. However, the same study shows no difference in DHA content of the fasting plasma, probably because the 5-day intervention in rats was not long enough to modify the fatty acid profile of phospholipids [66].
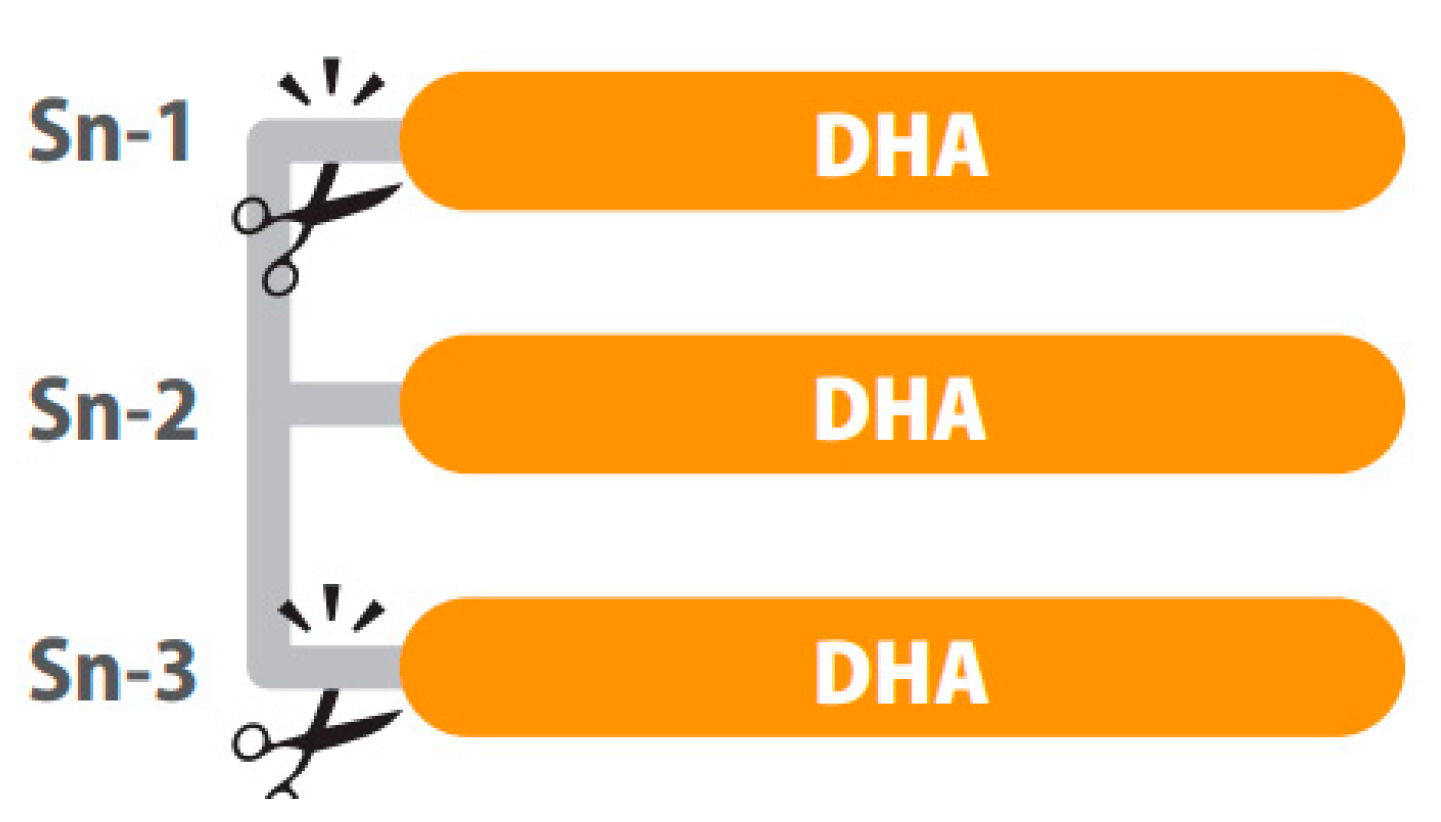
Figure 1. A triglyceride of DHA digested by lipases. The sn-1 and sn-3 positions of the glycerol backbone are cleaved while the fatty acids of the sn-2 position remains. Image courtesy of Brudylab®.
In addition to differences in the absorption, there are also divergences in the effect on the cells despite structured ω-3 LCPUFAs share common mechanisms with EE forms as schematically represented in Figure 2. In vitro studies using DHA-TG showed powerful antioxidant responses in comparison with EE-DHA in experiments with human fibroblasts and human retinal pigment epithelium cells through enhancing GSH synthesis significantly [17]. As mentioned before, some experimental studies have provided evidence about the interrelationship between DHA and GSH mainly through the regulation of nuclear factors like NFE2L2 [67][68], however TG-DHA seems to stimulate this reported pathway stronger than EE-DHA [17][69]. Moreover, a recent clinical trial proven significant anti-inflammatory properties after supplementation with TG-DHA through reduction of proinflammatory cytokines levels in patients with recurrent uveitis, one of the major causes of vision loss [57].
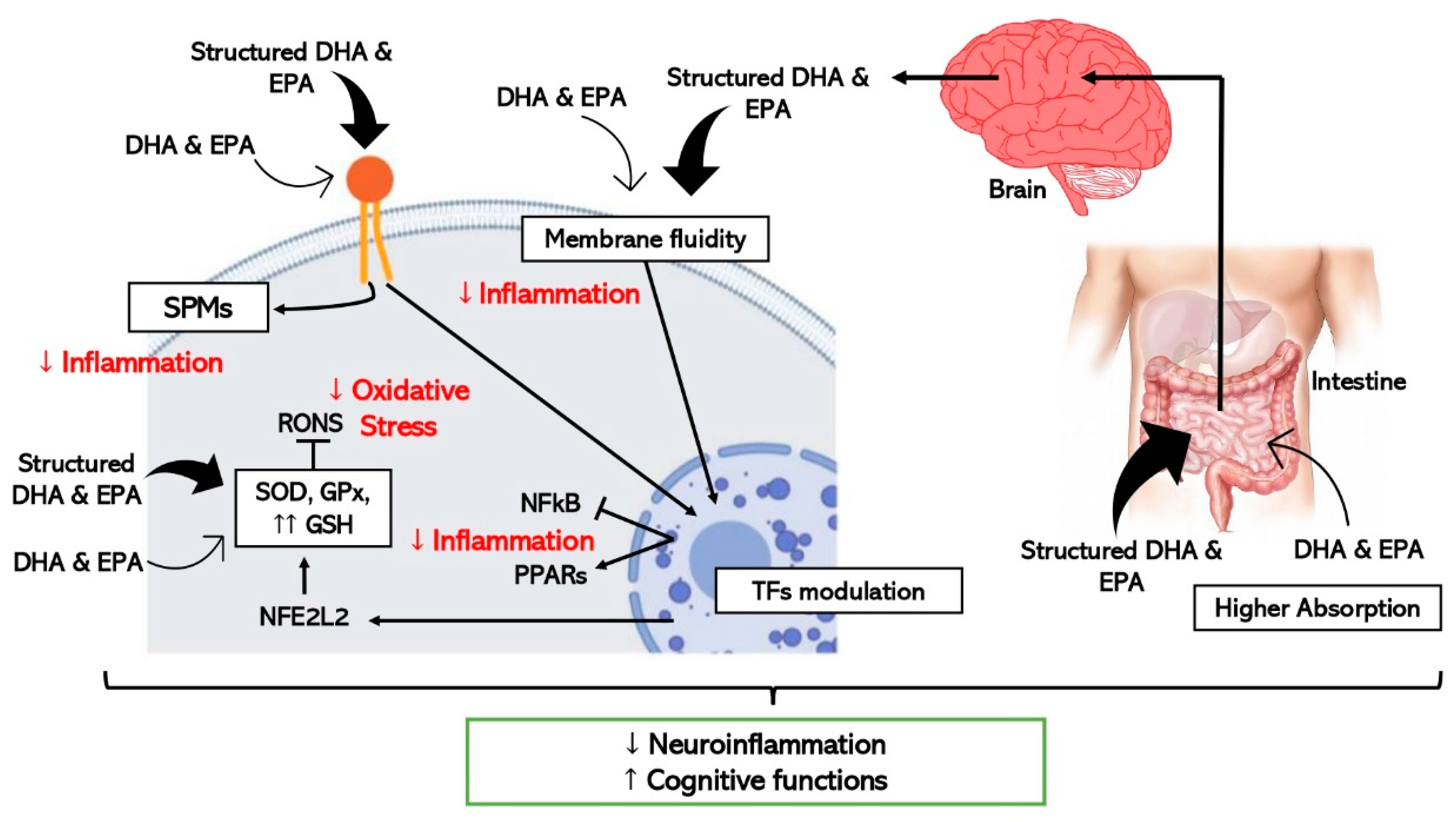
Figure 2. Schematic representation of the hypothetic mechanisms through which ω-3 LCPUFAs reduce neuroinflammation and improve cognitive function by modulating inflammation and oxidative stress in brain cells. Thick arrows express the enhanced effect of the structured forms of DHA and EPA (TFs, transcription factors).
Concerning neuroinflammation and cognitive decline, in vitro and pre-clinical studies show improved efficacy when structured ω-3 LCPUFAs are used in comparison with EE [64] as detailed in Figure 2. One study where the effect of TG-DHA on microglial activation was assessed and compared with EE-DHA, proved that TG-DHA treatment protected microglia cells from oxidative stress toxicity by attenuating nitric oxide (NO) production and suppressing the induction of inflammatory cytokines [70]. Furthermore, in the same study, when 50 or 250 mg/kg of TG-DHA was given orally to mice with autoimmune encephalomyelitis for a total of 56 days, a significant amelioration of the course and severity of the disease as compared to untreated animals was observed, concluding that TG-DHA is a promising nutritional immunomodulating agent in neuroinflammatory processes [70]. Supporting this data, another study using a Parkinsonism murine model, which constitutes a powerful neurotoxic model, indicated that 250 mg/kg of TG-DHA for 22 consecutive days acted as a neuroprotective agent and may constitute a promising therapeutic adjuvant [71].
Looking for a greater bioactive and targeted effect, other types of structured ω-3 LCPUFA, in addition to PL and TG, have been tested in pre-clinical studies. This is the case with ω-3 LCPUFAs esterified with lysophosphatidyl-choline (LPC). When LPC is esterified with a saturated fatty acid it becomes a highly proinflammatory molecule, while esterification with a ω-3 PUFA, causes it to have the opposite role [72]. LPC has a great affinity for the receptor MFSD2A (sodium-dependent LPC symporter 1), a transmembrane transporter of long-chain fatty acids across the blood brain barrier (BBB) [73], located exclusively on the luminal membrane of endothelial cells that line the blood vessels in the brain [74]. Consequently, in vivo studies showed greater brain uptake of DHA and higher DHA enrichment of cell membranes in neural tissue when LPC-DHA was supplemented in comparison with PL and TG-DHA [75][76].
Another example of a special ω-3 LCPUFA is AceDoPC® [77]. It is a structured DHA-PL acetylated at the sn-1 position—structurally similar to LPC-DHA—targeted to improve brain DHA levels. In an experimental ischemic stroke rat model, the intravenous injection of AceDoPC® proved to have more powerful anti-inflammatory effects, attenuating induced neuroinflammation by decreasing IL-6 production [64], in addition to exerting more neuroprotective effects than DHA-EE in another study with a stroke rat model [78]. Moreover, a study with neural stem progenitor cells (NSPCs) derived from the adult mouse brain showed enhanced neurogenesis with AceDoPC® over DHA-EE, especially under hypoxigenic (ischemic) conditions in vitro [79].
Furthermore, supplementation with synaptamide, which is mentioned as a bioactive form of DHA in the brain, has been assayed by Tyrtyshnaia et al. using synpatamide extracted from squid and administered subcutaneously to rats as a water emulsion. Synaptamide treatment attenuated microglial activation, release of proinflammatory cytokines, and decreased hippocampal neurogenesis in rats with a sciatic nerve chronic constriction injury [80].
Studies supplementing isolated forms of SPMs, derived lipid mediators of ω-3 LCPUFA, have been performed. Although limited literature relates the anti-inflammatory effect of SPMs with cognitive decline improvement [44], successful results have been observed with the use of supplements with SPMs and precursors of SPMs. It has been proved that supplementation with an oil enriched with SPMs and precursors, significantly increases SPM concentration in peripheral blood in humans [81], and larger intervention studies have been performed demonstrating that an orally administered SPM-enriched supplement improved the quality of life and reduced pain in a sample of adults with chronic pain [82].
Considering all the favourable literature, research to test the effect on brain health of new structures of ω-3 LCPUFA must go further to gain knowledge about the promising effects of targeting the positional distribution of ω-3 LCPUFA, and to find new chemical and enzymatic modification strategies to design special structures with targeted effects.
6. High-Throughput Techniques and Biological Models to Study Omega-3 LCPUFAs and Cognitive Decline
To discover the real potential of the ω-3 LCPUFAs on healthy aging not all experimental procedures can be entirely focused on nutritional interventions. Specific mechanisms must be elucidated, and fast screening methods for new structures of ω-3 LCPUFA are needed. The following points suggest high-throughput techniques and models that provide large information in a fast and cost-effective manner that might be very helpful in the study of ω-3 LCPUFAs.
6.1. “Omic” Technologies
Thanks to innovative breakthroughs in genome sequencing, bioinformatics, and analytic tools such as liquid (LC) and gas (GC) chromatography, mass spectrometry (MS), and nuclear magnetic resonance (NMR), “omics” technologies have appeared: genomics, transcriptomics, proteomics, metabolomics, metagenomics and epigenomics [83]. They are based on high-throughput identification and quantification of small and large molecules in cells, tissues, and biofluids, and are a powerful tool for mapping global biochemical changes and discovery of biomarkers [84]. Many omics disciplines are employed in food and nutrition research, and it is a prevalent recognition among food scientist that omics-based approaches are highly effective when they are exploited properly [85].
There are different motivations for conducting omic research, but commonly, they are performed to obtain a comprehensive understanding of the biological system under study, or to associate the omics-based molecular measurements with a clinical outcome of interest [86]. Researchers now put the combination of multiple omics analyses (integrated omics) into practice to exhaustively understand the functionality of food components of which nuclear NMR and MS are major choices. Generally, NMR is easier to perform and applicable to a wider range of compounds, although it is less sensitive compared to MS-based techniques. In contrast, GC or LC are used depending on the property of the target molecules [85].
The development of omic technologies brought about the expectation that an exhaustive molecular description of aging-regulated processes should have been possible, thereby shedding light on its mechanisms [87]. They would provide fast and precise information of specific and early biomarkers of the onset of homeostatic disturbances while aging, and this could help translational clinical research to describe quantitatively or qualitatively the health status of an individual or underlying aging mechanism [88][89]. For example, mass spectrometry-based omic technologies were used to reveal metabolic changes taking place during normal brain aging: metabolomic and proteomic analyses of different regions of mouse brain during the adult lifespan demonstrated an energy metabolic drift or significant imbalance in core metabolite levels in the aged animals [90].
6.2. Fast and Cost-Effective Experimental Models
The place of animals in our modern societies, especially of mammals, is often debated, particularly the right to use mammals to benefit human purposes when there is the possibility that they will be harmed. Moreover, not all results obtained from animals, mainly rodents, can be directly translated to humans, although there are remarkable anatomical and physiological similarities [94]. Consequently, more researchers have started to use alternative in vivo models, such as invertebrates or fish, rather than mammals which are between in vitro and rodent models, to obtain information from a complete organism in a fast and cost-effective manner. They are especially useful for the study of regulatory pathways and cellular mechanisms, as well as being suitable as screening platforms to test drugs and bioactive compounds. In addition, since the food industry is getting more and more involved in health issues by designing innovative foods that contribute to a better nutritional profile or to a certain functionality, fast and cost-effective models can be used to pre-screen compounds with bioactivity to speed up the demonstration of active ingredient effectiveness [95]. As represented in Figure 3, the simultaneous combination of these sorts of biological models with a biology systems approach using omic technologies, will make it easier and more feasible to scale-up the business pipeline of a bioactive ingredient until scientific and legal requirements, in terms of demonstrating efficacy are met.
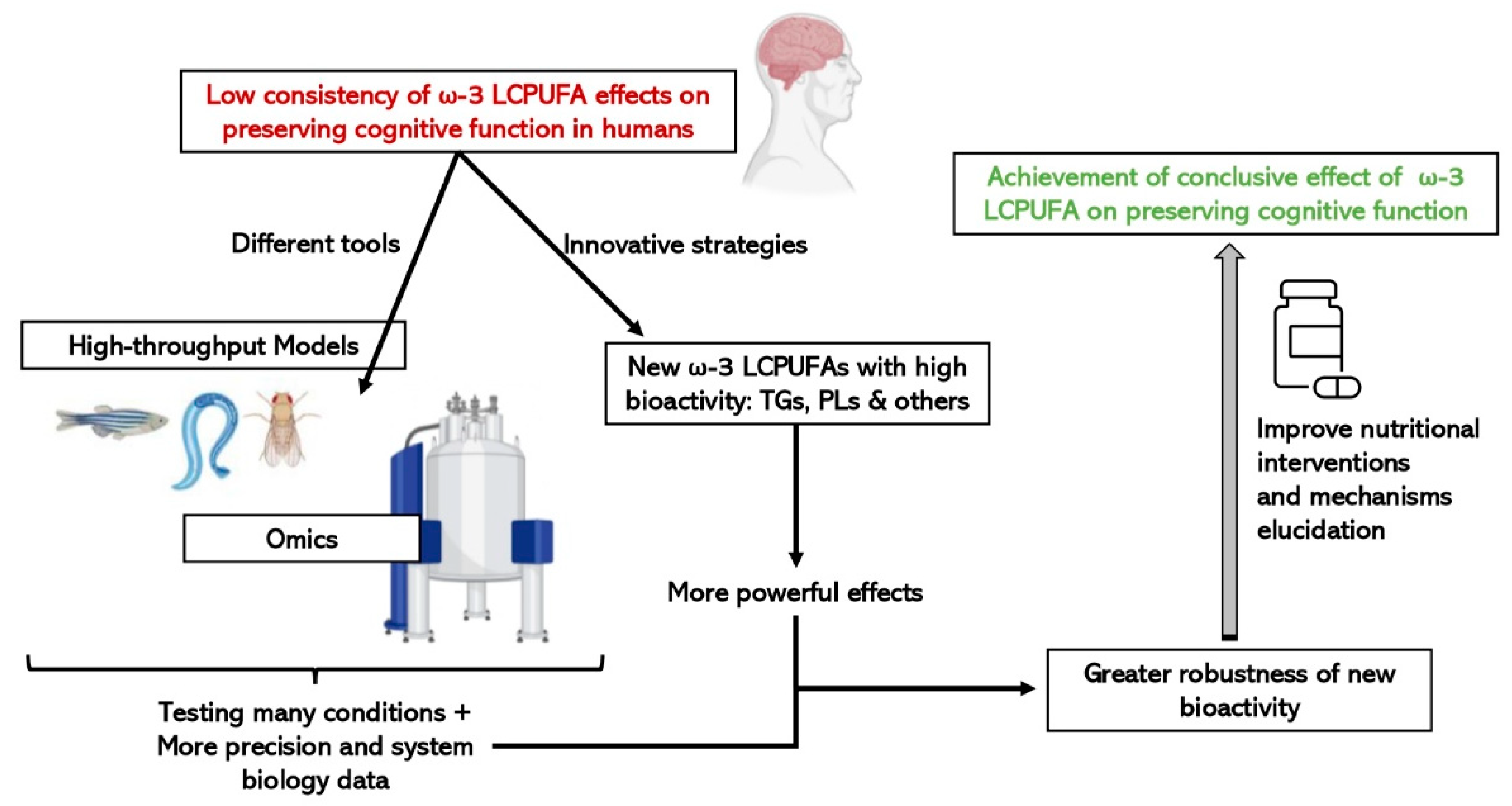
Figure 3. Advantages of integrating the use of omic technologies and small high-throughput in vivo models on the study of the efficacy of new structures of ω-3 LCPUFAs on cognitive function.
This entry is adapted from the peer-reviewed paper 10.3390/ijms23073472
References
- Haveman-Nies, A.; De Groot, L.C.P.G.M.; Van Staveren, W.A. Dietary quality, lifestyle factors and healthy ageing in Europe: The SENECA study. Age Ageing 2003, 32, 427–434.
- Tucker-Drob, E.M. Cognitive Aging and Dementia: A Life Span Perspective. Annu. Rev. Dev. Psychol. 2019, 1, 177.
- Mantzorou, M.; Vadikolias, K.; Pavlidou, E.; Tryfonos, C.; Vasios, G.; Serdari, A.; Giaginis, C. Mediterranean diet adherence is associated with better cognitive status and less depressive symptoms in a Greek elderly population. Aging Clin. Exp. Res. 2021, 33, 1033–1040.
- Herranz, N.; Gil, J. Mechanisms and functions of cellular senescence. J. Clin. Investig. 2018, 128, 1238–1246.
- Zuo, L.; Prather, E.R.; Stetskiv, M.; Garrison, D.E.; Meade, J.R.; Peace, T.I.; Zhou, T. Molecular Sciences Inflammaging and Oxidative Stress in Human Diseases: From Molecular Mechanisms to Novel Treatments. Int. J. Mol. Sci. 2019, 20, 4472.
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757.
- Ruthsatz, M.; Candeias, V. Non-communicable disease prevention, nutrition and aging. Acta Biomed 2020, 91, 379–388.
- Ferrari, C.K.B. Current opinion Functional foods and physical activities in health promotion of aging people. Maturitas 2007, 58, 327–339.
- García-Esquinas, E.; Ortolá, R.; Banegas, J.R.; Lopez-García, E.; Rodríguez-Artalejo, F. Dietary n-3 polyunsaturated fatty acids, fish intake and healthy ageing. Int. J. Epidemiol. 2019, 48, 1914–1924.
- Scarmeas, N.; Anastasiou, C.A.; Yannakoulia, M. Nutrition and prevention of cognitive impairment. Lancet Neurol. 2018, 17.
- Kang, E.Y.; Kim, H.K.; Go, G.-W. Effective Nutraceuticals on Age-Associated Cognitive Decline: A Systematic Review. Curr. Dev. Nutr. 2021, 5, 24.
- Barnes, S.; Chowdhury, S.; Gatto, N.M.; Fraser, G.E.; Lee, G.J. Omega-3 fatty acids are associated with blood–brain barrier integrity in a healthy aging population. Brain Behav. 2021, 11, e2273.
- Yurko-Mauro, K.; Alexander, D.D.; Van Elswyk, M.E. Docosahexaenoic Acid and Adult Memory: A Systematic Review and Meta-Analysis. PLoS ONE 2015, 10, e0120391.
- Dyall, S.C. Long-chain omega-3 fatty acids and the brain: A review of the independent and shared effects of EPA, DPA and DHA. Front. Aging Neurosci. 2015, 7, 52.
- Troesch, B.; Eggersdorfer, M.; Laviano, A.; Rolland, Y.; Smith, A.D.; Warnke, I.; Weimann, A.; Calder, P.C. Expert Opinion on Benefits of Long-Chain Omega-3 Fatty Acids (DHA and EPA) in Aging and Clinical Nutrition. Nutrients 2020, 12, 2555.
- Balakrishnan, J.; Kannan, S.; Govindasamy, A. Structured form of DHA prevents neurodegenerative disorders: A better insight into the pathophysiology and the mechanism of DHA transport to the brain. Nutr. Res. 2021, 85, 119–134.
- Lafuente, M.; González-Herrero, M.E.R.; Villadóniga, S.R.; Domingo, J.C. Antioxidant Activity and Neuroprotective Role of Docosahexaenoic Acid (DHA) Supplementation in Eye Diseases That Can Lead to Blindness: A Narrative Review. Antioxidants 2021, 10, 386.
- Sharma, R. Bioactive food components for managing cellular senescence in aging and disease: A critical appraisal and perspectives. PharmaNutrition 2021, 18, 100281.
- Stark, K.D.; Van Elswyk, M.E.; Higgins, M.R.; Weatherford, C.A.; Salem, N. Global survey of the omega-3 fatty acids, docosahexaenoic acid and eicosapentaenoic acid in the blood stream of healthy adults. Prog. Lipid Res. 2016, 63, 132–152.
- Swanson, D.; Block, R.; Mousa, S.A. Omega-3 Fatty Acids EPA and DHA: Health Benefits throughout Life. Adv. Nutr. 2012, 3, 1.
- Prince, M.; Wimo, A.; Guerchet, M.; Ali, G.-C.; Wu, Y.-T.; Prina, M. World Alzheimer Report 2015. The Global Impact of Dementia; Alzheimer’s Disease International: London, UK, 2015; Available online: https://www.alzint.org/resource/world-alzheimer-report-2015/ (accessed on 24 February 2022).
- Chataigner, M.; Mortessagne, P.; Lucas, C.; Pallet, V.; Layé, S.; Mehaignerie, A.; Bouvret, E.; Dinel, A.L.; Joffre, C. Dietary fish hydrolysate supplementation containing n-3 LC-PUFAs and peptides prevents short-term memory and stress response deficits in aged mice. Brain. Behav. Immun. 2021, 91, 716–730.
- Fiala, M.; Terrando, N.; Dalli, J. Specialized Pro-Resolving Mediators from Omega-3 Fatty Acids Improve Amyloid-β Phagocytosis and Regulate Inflammation in Patients with Minor Cognitive Impairment. J. Alzheimer’s Dis. 2015, 48, 293–301.
- Cutuli, D. Functional and Structural Benefits Induced by Omega-3 Polyunsaturated Fatty Acids during Aging. Curr. Neuropharmacol. 2017, 15, 534.
- Smith, A.D.; Refsum, H.; Bottiglieri, T.; Fenech, M.; Hooshmand, B.; McCaddon, A.; Miller, J.W.; Rosenberg, I.H.; Obeid, R. Homocysteine and Dementia: An International Consensus Statement. J. Alzheimer’s Dis. 2018, 62, 561–570.
- Scientific Opinion on Dietary Reference Values for folate. EFSA J. 2014, 12.
- Durga, J.; van Boxtel, M.P.; Schouten, E.G.; Kok, F.J.; Jolles, J.; Katan, M.B.; Verhoef, P. Effect of 3-year folic acid supplementation on cognitive function in older adults in the FACIT trial: A randomised, double blind, controlled trial. Lancet 2007, 369, 208–216.
- Mecocci, P.; Boccardi, V.; Cecchetti, R.; Bastiani, P.; Scamosci, M.; Ruggiero, C.; Baroni, M. A Long Journey into Aging, Brain Aging, and Alzheimer’s Disease Following the Oxidative Stress Tracks. J. Alzheimer’s Dis. 2018, 62, 1319–1335.
- Zandi, P.P.; Anthony, J.C.; Khachaturian, A.S.; Stone, S.V.; Gustafson, D.; Tschanz, J.A.T.; Norton, M.C.; Welsh-Bohmer, K.A.; Breitner, J.C.S. Reduced risk of Alzheimer disease in users of antioxidant vitamin supplements: The Cache County Study. Arch. Neurol. 2004, 61, 82–88.
- Simpson, E.E.A.; Maylor, E.A.; Rae, G.; Meunier, N.; Andriollo-Sanchez, M.; Catasta, G.; McConville, C.; Ferry, M.; Polito, A.; Stewart-Knox, B.J.; et al. Cognitive function in healthy older European adults: The ZENITH study. Eur. J. Clin. Nutr. 2005, 59, S26–S30.
- Feart, C.; Letenneur, L.; Helmer, C.; Samieri, C.; Schalch, W.; Etheve, S.; Delcourt, C.; Dartigues, J.F.; Barberger-Gateau, P. Plasma Carotenoids Are Inversely Associated With Dementia Risk in an Elderly French Cohort. J. Gerontol. Ser. A 2016, 71, 683–688.
- Kesse-Guyot, E.; Fezeu, L.; Andreeva, V.A.; Touvier, M.; Scalbert, A.; Hercberg, S.; Galan, P. Total and specific polyphenol intakes in midlife are associated with cognitive function measured 13 years later. J. Nutr. 2012, 142, 76–83.
- Devore, E.E.; Kang, J.H.; Breteler, M.M.B.; Grodstein, F. Dietary intakes of berries and flavonoids in relation to cognitive decline. Ann. Neurol. 2012, 72, 135–143.
- Nooyens, A.C.J.; Milder, I.E.J.; Van Gelder, B.M.; Bueno-De-Mesquita, H.B.; Van Boxtel, M.P.J.; Verschuren, W.M.M. Diet and cognitive decline at middle age: The role of antioxidants. Br. J. Nutr. 2015, 113, 1410–1417.
- Nadeem, M.S.; Kazmi, I.; Ullah, I.; Muhammad, K.; Anwar, F. Allicin, an Antioxidant and Neuroprotective Agent, Ameliorates Cognitive Impairment. Antioxidants 2021, 11, 87.
- Danthiir, V.; Hosking, D.E.; Nettelbeck, T.; Vincent, A.D.; Wilson, C.; O’callaghan, N.; Calvaresi, E.; Clifton, P.; Wittert, G.A. An 18-mo randomized, double-blind, placebo-controlled trial of DHA-rich fish oil to prevent age-related cognitive decline in cognitively normal older adults. Am. J. Clin. Nutr. 2018, 107, 754–756.
- Zhang, X.W.; Hou, W.S.; Li, M.; Tang, Z.Y. Omega-3 fatty acids and risk of cognitive decline in the elderly: A meta-analysis of randomized controlled trials. Aging Clin. Exp. Res. 2016, 28, 165–166.
- Lee, J.W.; Huang, B.X.; Kwon, H.S.; Rashid, M.A.; Kharebava, G.; Desai, A.; Patnaik, S.; Marugan, J.; Kim, H.Y. Orphan GPR110 (ADGRF1) targeted by N-docosahexaenoylethanolamine in development of neurons and cognitive function. Nat. Commun. 2016, 7, 13123.
- Kim, H.Y.; Moon, H.S.; Cao, D.; Lee, J.; Kevala, K.; Jun, S.B.; Lovinger, D.M.; Akbar, M.; Huang, B.X. N-docosahexaenoylethanolamide promotes development of hippocampal neurons. Biochem. J. 2011, 435, 327–336.
- Kim, H.Y.; Spector, A.A. N-Docosahexaenoylethanolamine: A neurotrophic and neuroprotective metabolite of docosahexaenoic acid. Mol. Asp. Med. 2018, 64, 34–44.
- Choi, Y.; Kim, H.S.; Shin, K.Y.; Kim, E.M.; Kim, M.; Kim, H.S.; Park, C.H.; Jeong, Y.H.; Yoo, J.; Lee, J.P.; et al. Minocycline Attenuates Neuronal Cell Death and Improves Cognitive Impairment in Alzheimer’s Disease Models. Neuropsychopharmacology 2007, 32, 2393–2404.
- Butler, M.J.; Deems, N.P.; Muscat, S.; Butt, C.M.; Belury, M.A.; Barrientos, R.M. Dietary DHA prevents cognitive impairment and inflammatory gene expression in aged male rats fed a diet enriched with refined carbohydrates. Brain. Behav. Immun. 2021, 98, 198–209.
- Joffre, C.; Dinel, A.L.; Chataigner, M.; Pallet, V.; Layé, S. n-3 Polyunsaturated Fatty Acids and Their Derivates Reduce Neuroinflammation during Aging. Nutrients 2020, 12, 647.
- Krashia, P.; Cordella, A.; Nobili, A.; La Barbera, L.; Federici, M.; Leuti, A.; Campanelli, F.; Natale, G.; Marino, G.; Calabrese, V.; et al. Blunting neuroinflammation with resolvin D1 prevents early pathology in a rat model of Parkinson’s disease. Nat. Commun. 2019, 10, 3945.
- Tan, A.; Sullenbarger, B.; Prakash, R.; McDaniel, J.C. Supplementation with eicosapentaenoic acid and docosahexaenoic acid reduces high levels of circulating proinflammatory cytokines in aging adults: A randomized, controlled study. Prostaglandins. Leukot. Essent. Fatty Acids 2018, 132, 23–29.
- Malik, A.; Ramadan, A.; Vemuri, B.; Siddiq, W.; Amangurbanova, M.; Ali, A.; Welty, F.K. ω-3 Ethyl ester results in better cognitive function at 12 and 30 months than control in cognitively healthy subjects with coronary artery disease: A secondary analysis of a randomized clinical trial. Am. J. Clin. Nutr. 2021, 113, 1168–1176.
- Kuszewski, J.C.; Howe, P.R.C.; Wong, R.H.X. Evaluation of Cognitive Performance following Fish-Oil and Curcumin Supplementation in Middle-Aged and Older Adults with Overweight or Obesity. J. Nutr. 2020, 150, 3190–3199.
- Mcnamara, R.K.; Kalt, W.; Shidler, M.D.; Mcdonald, J.; Summer, S.S.; Stein, A.L.; Stover, A.N.; Krikorian, R. Cognitive response to fish oil, blueberry, and combined supplementation in older adults with subjective cognitive impairment. Neurobiol. Aging 2018, 64, 147–156.
- Balachandar, R.; Soundararajan, S.; Bagepally, B.S. Docosahexaenoic acid supplementation in age-related cognitive decline: A systematic review and meta-analysis. Eur. J. Clin. Pharmacol. 2020, 76, 639–648.
- Sydenham, E.; Dangour, A.D.; Lim, W.S. Omega 3 fatty acid for the prevention of cognitive decline and dementia. Cochrane Database Syst. Rev. 2012, 130, 419.
- Van De Rest, O.; Geleijnse, J.M.; Kok, F.J.; Van Staveren, W.A.; Dullemeijer, C.; OldeRikkert, M.G.M.; Beekman, A.T.F.; De Groot, C.P.G.M. Effect of fish oil on cognitive performance in older subjects. Neurology 2008, 71, 430–438.
- Baleztena, J.; Ruiz-Canela, M.; Sayon-Orea, C.; Pardo, M.; Añorbe, T.; Gost, J.I.; Gomez, C.; Ilarregui, B.; Bes-Rastrollo, M. Association between cognitive function and supplementation with omega-3 PUFAs and other nutrients in ≥ 75 years old patients: A randomized multicenter study. PLoS ONE 2018, 13, e0193568.
- Canhada, S.; Castro, K.; Perry, I.S.; Luft, V.C. Omega-3 fatty acids’ supplementation in Alzheimer’s disease: A systematic review. Nutr. Neurosci. 2017, 21, 529–538.
- Van Soest, A.P.M.; Van De Rest, O.; Witkamp, R.F.; De Groot, L.C.P.G.M. Positive effects of folic acid supplementation on cognitive aging are dependent on ω-3 fatty acid status: A post hoc analysis of the FACIT trial. Am. J. Clin. Nutr. 2021, 113, 801–809.
- Scarmeas, N. State of science in nutrition clinical trials for dementia prevention. Alzheimer’s Dement. 2021, 17, e049654.
- Bandarra, N.M.; Lopes, P.A.; Martins, S.V.; Ferreira, J.; Alfaia, C.M.; Rolo, E.A.; Correia, J.J.; Pinto, R.M.A.; Ramos-Bueno, R.P.; Batista, I.; et al. Docosahexaenoic acid at the sn-2 position of structured triacylglycerols improved n-3 polyunsaturated fatty acid assimilation in tissues of hamsters. Nutr. Res. 2016.
- Pinazo-Durán, M.D.; García-Medina, J.J.; Sanz-González, S.M.; O’connor, J.E.; Casaroli-Marano, R.P.; Valero-Velló, M.; López-Gálvez, M.; Peris-Martínez, C.; Zanón-Moreno, V.; Diaz-Llopis, M. Signature of Circulating Biomarkers in Recurrent Non-Infectious Anterior Uveitis. Immunomodulatory Effects of DHA-Triglyceride. A Pilot Study. Diagnostics 2021, 11, 724.
- Chang, M.; Yang, J.; Guo, X.; Zhang, T.; Liu, R.; Jin, Q.; Wang, X. Medium/long-chain structured triglycerides are superior to physical mixtures triglycerides in Caenorhabditis elegans lifespan through an AMPK modified pathway. Food Biosci. 2021, 39, 2212–4292.
- Neubronner, J.; Schuchardt, J.P.; Kressel, G.; Merkel, M.; Von Schacky, C.; Hahn, A. Enhanced increase of omega-3 index in response to long-term n-3 fatty acid supplementation from triacylglycerides versus ethyl esters. Eur. J. Clin. Nutr. 2010, 65, 247–254.
- Kroupova, P.; Keijer, J.; Bunschoten, A.; Vodicka, M.; Irodenko, I.; Oseeva, M.; Zacek, P.; Kopecky, J.; Rossmeisl, M.; Horakova, O. Omega-3 Phospholipids from Krill Oil Enhance Intestinal Fatty Acid Oxidation More Effectively than Omega-3 Triacylglycerols in High-Fat Diet-Fed Obese Mice. Nutrients 2020, 12, 2037.
- Dyerberg, J.; Madsen, P.; Møller, J.M.; Aardestrup, I.; Schmidt, E.B. Bioavailability of marine n-3 fatty acid formulations. Prostaglandins Leukot. Essent. Fat. Acids 2010, 83, 137–141.
- Kitson, A.P.; Metherel, A.H.; Chen, C.T.; Domenichiello, A.F.; Trépanier, M.O.; Berger, A.; Bazinet, R.P. Effect of dietary docosahexaenoic acid (DHA) in phospholipids or triglycerides on brain DHA uptake and accretion. J. Nutr. Biochem. 2016, 33, 91–102.
- Wen, M.; Zhao, Y.; Shi, H.; Wang, C.; Zhang, T.; Wang, Y.; Xue, C. Short-term supplementation of DHA as phospholipids rather than triglycerides improve cognitive deficits induced by maternal omega-3 PUFA deficiency during the late postnatal stage. Food Funct. 2021, 12, 564–572.
- Fourrier, C.; Remus-Borel, J.; Greenhalgh, A.D.; Guichardant, M.; Bernoud-Hubac, N.; Lagarde, M.; Joffre, C.; Layé, S. Docosahexaenoic acid-containing choline phospholipid modulates LPS-induced neuroinflammation in vivo and in microglia in vitro. J. Neuroinflamm. 2017, 14, 170.
- Chouinard-Watkins, R.; Lacombe, R.J.S.; Metherel, A.H.; Masoodi, M.; Bazinet, R.P. DHA Esterified to Phosphatidylserine or Phosphatidylcholine is More Efficient at Targeting the Brain than DHA Esterified to Triacylglycerol. Mol. Nutr. Food Res. 2019, 63, 1801224.
- Linderborg, K.M.; Kulkarni, A.; Zhao, A.; Zhang, J.; Kallio, H.; Magnusson, J.D.; Haraldsson, G.G.; Zhang, Y.; Yang, B. Bioavailability of docosahexaenoic acid 22:6(n-3) from enantiopure triacylglycerols and their regioisomeric counterpart in rats. Food Chem. 2019.
- Di Nunzio, M.; Valli, V.; Bordoni, A. PUFA and oxidative stress. Differential modulation of the cell response by DHA. Int. J. Food Sci. Nutr. 2016, 67, 834–843.
- Arab, K.; Rossary, A.; Flourié, F.; Tourneur, Y.; Steghens, J.-P. Docosahexaenoic acid enhances the antioxidant response of human fibroblasts by upregulating γ-glutamyl-cysteinyl ligase and glutathione reductase. Br. J. Nutr. 2006, 95, 18–26.
- Domingo, J.C.; Villegas, J.A. Use of DHA for treating a pathology associated with cellular oxidative damage. World Intellect. Prop. Organ. 2007, 100.
- Mancera, P.; Wappenhans, B.; Cordobilla, B.; Virgili, N.; Pugliese, M.; Rueda, F.; Espinosa-Parrilla, J.F.; Domingo, J.C. Natural Docosahexaenoic Acid in the Triglyceride Form Attenuates In Vitro Microglial Activation and Ameliorates Autoimmune Encephalomyelitis in Mice. Nutrients 2017, 9, 681.
- Gómez-Soler, M.; Cordobilla, B.; Morató, X.; Fernández-Dueñas, V.; Domingo, J.C.; Ciruela, F. Triglyceride form of docosahexaenoic acid mediates neuroprotection in experimental parkinsonism. Front. Neurosci. 2018, 12.
- Liu, P.; Zhu, W.; Chen, C.; Yan, B.; Zhu, L.; Chen, X.; Peng, C. The mechanisms of lysophosphatidylcholine in the development of diseases. Life Sci. 2020, 247.
- Nguyen, L.N.; Ma, D.; Shui, G.; Wong, P.; Cazenave-Gassiot, A.; Zhang, X.; Wenk, M.R.; Goh, E.L.K.; Silver, D.L. Mfsd2a is a transporter for the essential omega-3 fatty acid docosahexaenoic acid. Nature 2014, 509, 503–506.
- Semba, R.D. Perspective: The Potential Role of Circulating Lysophosphatidylcholine in Neuroprotection against Alzheimer Disease. Adv. Nutr. 2020, 11, 760.
- Sugasini, D.; Yalagala, P.C.R.; Goggin, A.; Tai, L.M.; Subbaiah, P.V. Enrichment of brain docosahexaenoic acid (DHA) is highly dependent upon the molecular carrier of dietary DHA: Lysophosphatidylcholine is more efficient than either phosphatidylcholine or triacylglycerol. J. Nutr. Biochem. 2019, 74, 108231.
- Sugasini, D.; Yalagala, P.C.R.; Subbaiah, P.V. Efficient enrichment of retinal DHA with dietary lysophosphatidylcholine-DHA: Potential application for retinopathies. Nutrients 2020, 12, 3114.
- Hachem, M.; Nacir, H.; Picq, M.; Belkouch, M.; Bernoud-Hubac, N.; Windust, A.; Meiller, L.; Sauvinet, V.; Feugier, N.; Lambert-Porcheron, S.; et al. Docosahexaenoic Acid (DHA) Bioavailability in Humans after Oral Intake of DHA-Containing Triacylglycerol or the Structured Phospholipid AceDoPC®. Nutrients 2020, 12, 251.
- Chauveau, F.; Cho, T.-H.; Perez, M.; Guichardant, M.; Riou, A.; Aguettaz, P.; Picq, M.; Lagarde, M.; Berthezene, Y.; Nighoghossian, N.; et al. Brain-Targeting Form of Docosahexaenoic Acid for Experimental Stroke Treatment: MRI Evaluation and Anti-Oxidant Impact. Curr. Neurovasc. Res. 2011, 8, 95–102.
- Lo Van, A.; Sakayori, N.; Hachem, M.; Belkouch, M.; Picq, M.; Fourmaux, B.; Lagarde, M.; Osumi, N.; Bernoud-Hubac, N. Targeting the Brain with a Neuroprotective Omega-3 Fatty Acid to Enhance Neurogenesis in Hypoxic Condition in Culture. Mol. Neurobiol. 2019, 56, 986–999.
- Tyrtyshnaia, A.A.; Egorova, E.L.; Starinets, A.A.; Ponomarenko, A.I.; Ermolenko, E.V.; Manzhulo, I.V. N-Docosahexaenoylethanolamine Attenuates Neuroinflammation and Improves Hippocampal Neurogenesis in Rats with Sciatic Nerve Chronic Constriction Injury. Mar. Drugs 2020, 18, 516.
- Souza, P.R.; Marques, R.M.; Gomez, E.A.; Colas, R.A.; De Matteis, R.; Zak, A.; Patel, M.; Collier, D.J.; Dalli, J. Enriched Marine Oil Supplements Increase Peripheral Blood Specialized Pro-Resolving Mediators Concentrations and Reprogram Host Immune Responses: A Randomized Double-Blind Placebo-Controlled Study. Circ. Res. 2020, 126, 75–90.
- Callan, N.; Hanes, D.; Bradley, R. Early evidence of efficacy for orally administered SPM-enriched marine lipid fraction on quality of life and pain in a sample of adults with chronic pain. J. Transl. Med. 2020, 18, 401.
- dos Santos, B.S.; da Silva, L.C.N.; da Silva, T.D.; Rodrigues, J.F.S.; Grisotto, M.A.G.; Dos Santos Correia, M.T.; Napoleão, T.H.; da Silva, M.V.; Paiva, P.M.G. Application of omics technologies for evaluation of antibacterial mechanisms of action of plant-derived products. Front. Microbiol. 2016, 7, 1466.
- Jiang, Y.; Zhu, Z.; Shi, J.; An, Y.; Zhang, K.; Wang, Y.; Li, S.; Jin, L.; Ye, W.; Cui, M.; et al. Metabolomics in the development and progression of dementia: A systematic review. Front. Neurosci. 2019, 13, 343.
- Kato, H.; Takahashi, S.; Saito, K. Omics and Integrated Omics for the Promotion of Food and Nutrition Science. J. Tradit. Complement. Med. 2011, 1, 25.
- Institute of Medicine. Evolution of Translational Omics: Lessons Learned and the Path Forward; Micheel, C.M., Nass, S.J., Omenn, G.S., Eds.; National Academies Press: Washington, DC, USA, 2012; ISBN 978-0-309-22418-5.
- Cellerino, A.; Ori, A. What have we learned on aging from omics studies? Semin. Cell Dev. Biol. 2017, 70, 177–189.
- Rivero-Segura, N.A.; Bello-Chavolla, O.Y.; Barrera-Vázquez, O.S.; Gutierrez-Robledo, L.M.; Gomez-Verjan, J.C. Promising biomarkers of human aging: In search of a multi-omics panel to understand the aging process from a multidimensional perspective. Ageing Res. Rev. 2020, 64, 101164.
- Wu, L.; Xie, X.; Liang, T.; Ma, J.; Yang, L.; Yang, J.; Li, L.; Xi, Y.; Li, H.; Zhang, J.; et al. Integrated Multi-Omics for Novel Aging Biomarkers and Antiaging Targets. Biomolecules 2021, 12, 39.
- Ivanisevic, J.; Stauch, K.L.; Petrascheck, M.; Benton, H.P.; Epstein, A.A.; Fang, M.; Gorantla, S.; Tran, M.; Hoang, L.; Kurczy, M.E.; et al. Metabolic drift in the aging brain. Aging 2016, 8, 1000–1020.
- Suárez, M.; Caimari, A.; Del Bas, J.M.; Arola, L. Metabolomics: An emerging tool to evaluate the impact of nutritional and physiological challenges. Trends Anal. Chem. 2017, 96, 79–88.
- Puiggròs, F.; Canela, N.; Arola, L. Metabolome responses to physiological and nutritional challenges This review comes from a themed issue on Foodomics technologies. Curr. Opin. Food Sci. 2015, 4, 111–115.
- Barberger-Gateau, P. Nutrition and brain aging: How can we move ahead? Eur. J. Clin. Nutr. 2014, 68, 1245–1249.
- Barré-Sinoussi, F.; Montagutelli, X. Animal models are essential to biological research: Issues and perspectives. Futur. Sci. 2015, 1.
- Caro, M.; Iturria, I.; Martinez-Santos, M.; Pardo, M.A.; Rainieri, S.; Tueros, I.; Navarro, V. Zebrafish dives into food research: Effectiveness assessment of bioactive compounds. Food Funct. 2016, 7, 2615–2623.
This entry is offline, you can click here to edit this entry!
