Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
As the principal representatives of thiopurines, 6-mercaptopurine (6MP) and its prodrug azathioprine (AZA) are primary immunomodulating agents. They are used for example to manage inflammatory bowel diseases (IBD), including ulcerative colitis (UC) and Crohn’s disease (CD), chronic inflammatory disorders of the gastrointestinal tract. Thiopurines were used to treat CD in the late 1960s and they are currently applied in around 60% of IBD patients.
- thiopurines
- azathioprine
- mercaptopurine
- thioguanine
- cytotoxicity
- inflammatory bowel disease
- Crohn’s disease
1. Metabolism of Thiopurine Drugs
Thiopurines undergo a complex metabolism, resulting in the formation of 6-thioguanine nucleotides (6TGN), the primary active therapeutic metabolite. Both azathioprine (AZA) and 6-mercaptopurine (6MP) , as prodrugs, undergo intracellular activation through a complex process, including the following enzymes: thiopurine methyltransferase (TPMT), xanthine oxidase/dehydrogenase (XOD), and hypoxanthine phosphoribosyltransferase (HPRT), forming three competitive pathways. In the first step, AZA is converted in the liver into 6MP, although only partly, by the enzyme glutathione S-transferase (GST), and evidence shows that it is also a non-enzymatic process [1][2]. Nevertheless, it is known that reduced activity of GST caused by mutations leads to reduced sensitivity to AZA in patients due to a lower concentration of active metabolites [3]. On the other hand, high activity of GST correlates with an increased risk of adverse effects and leukopenia during treatment with thiopurine drugs [4].
TPMT is responsible for the formation of inactive metabolites, 6-methylmercaptopurine (6MMP) and 6-methylmercaptopurine ribonucleotide (6MMPR), further converted to 6TGN by guanosine monophosphate synthetase (GMPS). However, it is worth noting that over 80% thiopurine-induced hepatotoxicity are due to a high concentration of 6-MMPR in one week after treatment initiation. In addition, elevated 6-MMPR levels can contribute to the explanation of gastrointestinal intolerance and general malaise, the most common limiting adverse events of thiopurines [5]. There is evidence that hepatotoxicity also correlates with elevated 6-MMP levels [6]. TPMT constitutes a crucial enzyme regulating the biotransformation of thiopurines. The genetic polymorphism influencing TPMT enzyme activity may result in a clinical response and possible myelotoxicity among IBD patients [7]. High enzymatic activity of TPMT leads to a low therapeutic 6TGN level [8][9]. At the same time, patients with low activity of TPMT are susceptible to the myelotoxicity of thiopurine therapy.
HPRT enzyme catalyzes the conversion of 6MP to 6-thioinosine monophosphate (6TIMP), which is then metabolized directly into active 6TGN using an inosine monophosphate dehydrogenase (IMPDH) or TPMT and GMPS pathway [10][11]. Xanthine oxidase/dehydrogenase (XOD), known as the cytoplasmic enzyme, is involved in the liver and intestinal degradation of endogenous and exogenous substrates such as thiopurine. XOD metabolizes an integral part of 6MP into inactive 6-thiouric acid (6TUA). The activity of XOD is also regulated by different single nucleotide polymorphisms located in the gene coding for this enzyme and may explain inter-individual variations in enzyme activity. Poor XOD metabolizers have an increased risk of thiopurine adverse effects, whereas rapid metabolizers are the group with an increased risk of thiopurine therapy failure [12][13]. A recent significant discovery regarding the metabolic pathway of thiopurine drugs is the enzyme NUDT15, which catalyzes the hydrolysis of 6-thioguanosine triphosphate (6TGTP) to 6-thioguanosine monophosphate (6TGMP) [14]. In 54 Japanese IBD patients (27 UC, 27 CD) showed that a NUDT15 mutation leading to increased deoksythioguanosine (dTG) of DNA-incorporated may be responsible for thiopurine-induced leukopenia through cell apoptosis [15].
2. Cytotoxic Properties and the Mechanism of Action of Thiopurines
Like immunosuppressants, the general action of thiopurines is based on the inactivation of the critical T-cell processes that lead to inflammation. However, the exact mechanism and effect of thiopurines is not yet fully understood, and their cytotoxicity is determined by a combination of different factors.
2.1. Induction of Cell Apoptosis
One of the primary therapeutic metabolites, 6TGN (more specifically 6-thioguanine triphosphate, 6TGTP), binds to Ras-related C3 botulinum toxin substrate 1 (Rac1) as a competitive antagonist, an intracellular enzyme, small GTPase, involved in the activation of the inflammatory cascade (including NF-κB and STAT-3 pathways), and finally stimulates apoptosis of gut T cells [16]. Essentially, AZA converts a costimulatory signal into an apoptotic signal through this binding process. However, the affinity of 6TGTP to Rac1 is lower than that of its standard binding partner GTP. This can explain the delayed onset of the clinical activity of AZA [17].
Furthermore, it is a known mechanism of apoptosis induction by thiopurines via a mitochondrial pathway. Incorporating TG into mitochondrial DNA (mtDNA) causes accumulation of oxidized TG, which inhibits transcription and translation, and finally results in loss of mitochondrial function. It is speculated that this mechanism influences toxicity and myopathy induced by thiopurines [18].
2.2. Inhibition of DNA Replication and RNA Transcription
There is evidence that deoxythioguanosine triphosphate (TdGTP), formed by ribonucleotide reductase from active metabolite 6TGTP during the biotransformation pathway, may be incorporated into DNA. TdGTP is a suitable substrate for DNA polymerases. Studies have shown that the incorporation frequency into the leukocyte DNA of patients undergoing treatment with MP varies between 1:32000 and 1:4000 thioguanine (TG) bases per guanine base [19][20]. In contrast, methylation of TG-thymine base pairs causes errors in the process of replication and transcription, also disrupting the DNA mismatch repair system, which results in DNA strand breaks, thus contributing to the thiopurine-mediated cytotoxicity effect. The studies also revealed that reactive oxygen generation and cell death are a consequence of TG incorporation into DNA [21][22].
During the incorporation of appropriate nucleotides into DNA, a particular role is played by the enzyme nudix hydrolase (nucleoside diphosphate-linked moiety X)-type motif 15 (NUDT15), which removes the wrong nucleotides from a cellular pole. NUDT15 can hydrolyze both TGTP and TdGTP. In vitro research has shown that NUDT15 knockdown results in significantly higher TGTP, TdGTP, and TG levels in DNA relative to controls [14][23]. Therefore, its role in the acute hematopoietic toxicity caused by thiopurine therapy has been proven.
2.3. Inhibition of De Novo Purine Synthesis
Thiopurines interfere with the synthesis of nucleic acids in dividing cells, acting mainly in the S phase. Mercaptopurine competes with hypoxanthine and guanine for hypoxanthine-guanyl phosphoribosyltransferase and is converted to thioinosine monophosphate (TIMP). This intracellular metabolite of mercaptopurine inhibits many reactions related to the metabolism of inosinic acid. S-methylation of TIMP with the participation of thiopurine S-methyltransferase (TPMT) leads to methylthioinosine monophosphate (MTIMP) formation. Both TIMP and MTIMP inhibit glutamine-5-phosphoribosyl pyrophosphate amidotransferase, the first enzyme in de novo synthesis of purine ribonucleotides [24].
Thiopurine metabolites vary in cytotoxicity, with methylthioinosine-mono-phosphate and thioguanosine-tri-phosphate being the most toxic and the methyl-thioguanosine nucleotides the least. Recent in vitro studies revealed that the most significant impact on cytotoxic properties of this group of drugs come from MTIMP and TG incorporation into DNA together as combined factors disturbing GTP signaling pathways [25].
3. Pharmacological Aspects and Clinical Characteristics of IBD Patients Treated with Thiopurines
Thiopurines are used as immunosuppressive drugs in both CD and UC. Guidelines for their application are set out in the European Crohn’s and Colitis Organisation (ECCO) Consensus [26][27].
3.1. Thiopurines in Crohn’s Disease (CD) Treatment
Experts suggest that thiopurines should not be used alone to induce remission in CD. Based on the available studies, the benefit of AZA monotherapy is not greater than that of a placebo [28]. However, the combination of thiopurines with infliximab (IFX) is recommended to induce remission in moderate to severe CD patients who have had an inadequate response to a conventional therapy and have not used AZA to date. Biologic and Immunomodulator Naive Patients in Crohn’s Disease (SONIC), which compared the efficacy of IFX monotherapy with IFX in combination with AZA in patients who failed to respond to glucocorticoid therapy, showed that combination therapy had higher rates of clinical remission and mucosal remission at week 26 compared with IFX monotherapy (RR: 1.64; 95% CI: 1.07–2.53) (RR: 1.82; 95% CI: 1.01–3.26) [29]. However, in clinical practice, where the patient uses thiopurines more often and the expected effect of maintaining remission is not achieved, which is recognized as an insufficient response to thiopurines. There are no reports that the combined therapy of thiopurines with IFX would be of benefit in achieving clinical remission in this group of patients, but doing so may be helpful in reducing IFX immunity. Naturally, it cannot forget about the immunogenicity of biological drugs. Therefore, in this aspect, the combined therapy should be considered by doctors individually for each patient [30].
Thiopurines have an undeniable and essential place in Crohn’s disease (CD) and are used to maintain remission in patients with a steroid-dependent form of the disease. Four hundred and eighty-nine patients were analyzed in the large meta-analysis of six studies. The advantage of AZA was demonstrated over the placebo in this group of patients (RR: 1.19; 95% CI: 1.05–1.34) [31]. However, it is not recommended to include thiopurines in all patients with newly diagnosed CD to maintain remission. It was suggested that an early introduction of these drugs may contribute to the favorable course of the disease. However, conducted research has failed to confirm this thesis. It was described by Panés et al., patients with a recent diagnosis of CD (<8 weeks) were randomized to two groups, one receiving a placebo and the other treated with AZA. At 76 weeks of therapy, no statistically significant differences were observed in the maintenance of disease remission between the two groups. The frequency of relapses and the need for glucocorticosteroids in both groups were comparable. Severe adverse effects occurred in 14 patients (20.6%) in the AZA group and 7 (11.1%) in the placebo group [32]. Its continuation is recommended in patients treated with thiopurines in long-term remission during thiopurine maintenance therapy. It is believed that there is a greater risk of the disease returning when treatment is discontinued [33].
3.2. Thiopurines in Ulcerative Colitis (UC) Treatment
In patients with steroid-dependent UC, thiopurine or IFX combined with thiopurine is also recommended. It was conducted by Ardizzone et al. showed that patients receiving prednisone at a dose of 40 mg/day and AZA at a quantity of 2 mg kg/day achieved significantly more steroid-free remission compared to the group receiving prednisone and 5-ASA 3.2 g per day (53% vs. 21%) [34]. Similarly, later research demonstrated an increase in remission without corticosteroids in UC patients treated with combination therapy after 16 weeks (39.7% vs. 22.1%, p = 0.017) [35]. Patients with UC display the same resistance to thiopurines as CD individuals. It is recommended to use biological therapy and, if IFX is used, it should be in combination with a thiopurine. Another indication for using thiopurines in mild to moderate UC would be patients who experience frequent relapses using mesalazine or steroids. Retrospective studies indicated that the remission rate in patients treated with AZA was 58% and increased to 87% after six months of dosing [36]. In patients with severe relapsing disease responding to steroids, cyclosporine, IFX with thiopurine should be considered for use in maintaining remission. In a retrospection analysis of 622 patients with CD and UC, the remission rate after six months of AZA use was 64% and 87%, respectively. After discontinuation of AZA, the proportion of patients remaining in remission after 1, 3, and 5 years was 0.63, 0.44, and 0.35, respectively. The duration of AZA treatment did not affect the relapse rate after treatment was discontinued [36].
3.3. Combination Therapy of Thiopurines and Infliximab in IBD
Clinical practice and research show that the combination of IFX and thiopurines is more effective in inducing remission in CD and UC than monotherapy with both agents. Data on other varieties of other biologics and thiopurines are either missing or contradictory [37]. The results of combined IFX-thiopurines therapy can be explained by the best-documented theory of reducing the risk of immunogenicity, which decreases the production of anti-IFX antibodies. This effect is related to greater availability of the drug and thus a far better response to therapy [29]. Anti-IFX formation was observed as early as 18 days after the initiation of treatment. Therefore, to limit their use, thiopurines should be administered as soon as possible [38].
3.4. Safety and Adverse Effects of Thiopurine Treatment
When deciding to use AZA for IBD treatment, it should always consider its long-term safety. Data from observational population studies suggest caution and regular monitoring, especially for the risk of skin non-melanoma neoplasms and lymphomas in patients exposed to long-term treatment with thiopurines [39]. Adverse effects were reported in 9.0% (22/245) of patients treated with thiopurines vs. 2.9% (9/311) in those treated with a placebo [26]. Inappropriate therapeutic levels of 6TGN may trigger toxicity at distinct levels, including hepatotoxicity, myleosupression, pancreatitis, or gastrointestinal intolerance [40] (Figure 1).
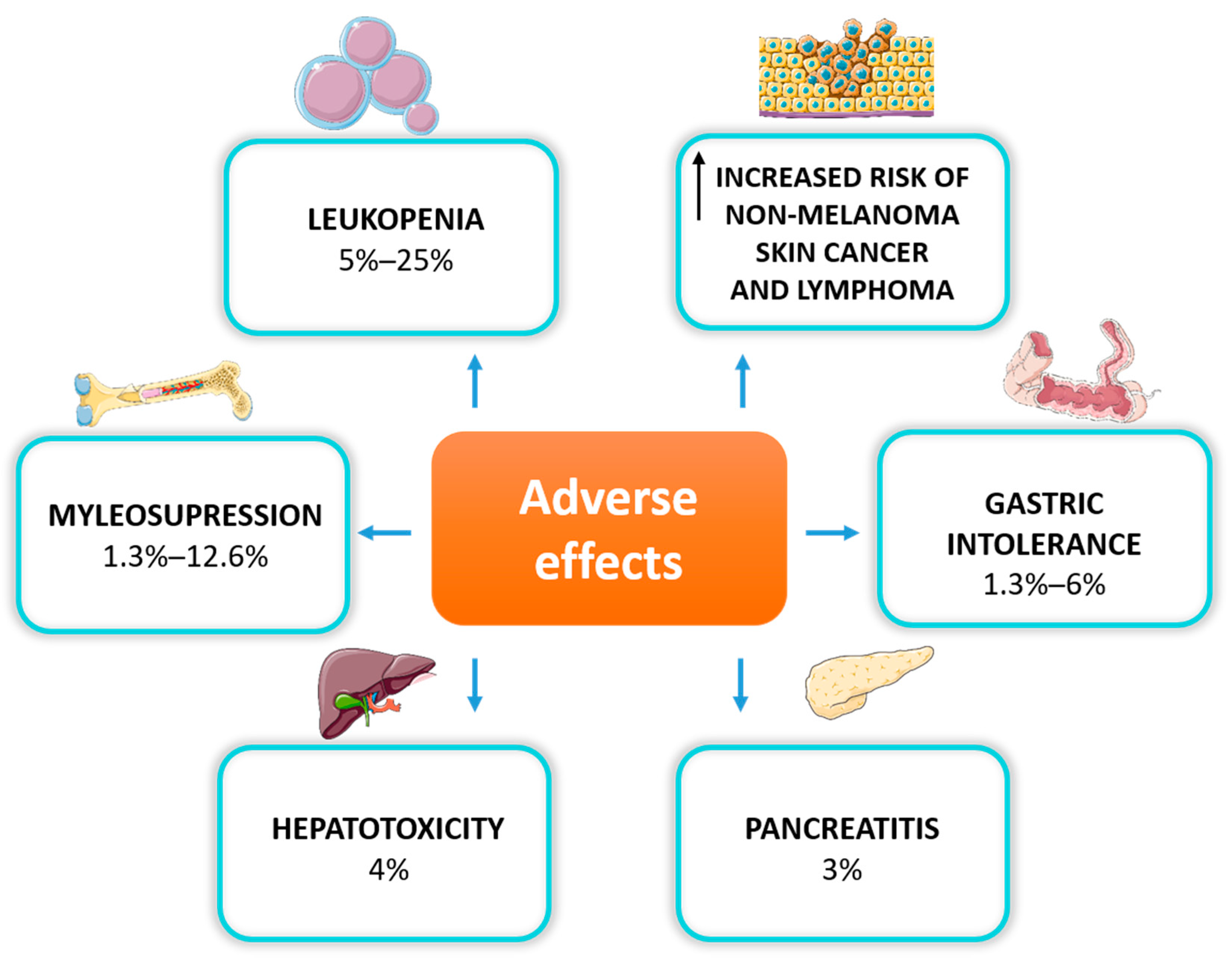
Figure 2. Adverse effects of thiopurine therapy in IBD patients.
The most commonly observed dose-dependent adverse reaction is myelosuppression, primarily manifested as leukopenia, which occurs in up to 20% of IBD patients due to TPMT gene polymorphism [41]. Thiopurines are also associated with myelosuppression, regardless of TPMT activity. Research has demonstrated that it can occur even many months after the initiation of the therapy, also in patients with normal TPMT phenotype [42]. Myelosuppression is the most severe hematological adverse drug reaction, leading to discontinuation of the treatment [43]. Thiopurines can also induce liver dysfunction by methylated intermediate metabolites, manifested as elevated liver enzymes, hepatitis, or hepatic veno-occlusive disease. Thiopurine-induced pancreatitis is another adverse effect of unknown origin, which occurs in less than 5% of patients treated with AZA or 6MP, mainly in the first month of treatment [44]. The most common reasons for thiopurine discontinuation are gastrointestinal disturbances such as nausea, vomiting and abdominal pain [45].
Much attention is paid to the adverse effects of combination therapy in IBD patients. Treatment with both IFX and thiopurines is known to generate specific adverse effects. Particular concerns stem from the risk of infection and cancer formation. Data on the safety of this form of treatment come mainly from a pooled post hoc analysis of drug registration trials, dedicated trials on combination therapy, and registries. It has been shown that patients treated with IFX in combination with immunosuppressants have a higher incidence of infections (120.07 (95% CI 110.66, 130.08)/100 patient-years vs. 92.47 (84.54, 100.94)/100 patient-years). Patients with CD treated with immunosuppressants had a higher incidence of malignancies compared to the absence of immunosuppression treatment (1.84 (0.22, 6.66)/100 patient-years vs. 0.00 (0.00, 0.00)/100 patient-years) [46].
Clinical registries are beneficial in assessing the safety of drugs. Firstly, they cover a larger group of patients compared to randomized trials. Secondly, they represent real clinical practice. The Crohn’s Therapy, Resource, Evaluation, and Assessment Tool (TREAT™) was designed to assess the safety of drugs in CD. This registry included 6273 patients. The median follow-up was six years. It showed that the use of immunosuppressants as monotherapy (OR 4.19; 95% CI 0.58–30.37; p = 0.16) or in combination with IFX (OR 3.33; 95% CI 0.46–24.06; p = 0.23) was associated with a numerically greater risk of malignancy than treatment with IFX alone (OR 1.96; 95% CI 0.23–17.02), although it was not statistically significant (p = 0.54) [47]. Similar results were obtained in other long-term studies of adalimumab (ADM) safety in CD [48]. The PYRAMID registry showed that ADM monotherapy vs.combination therapy showed a marked difference in the incidence of malignancies. In addition, there was a significant difference in the incidence of serious infections between ADM monotherapy and combination therapy (9.6 vs. 12.7%, p = 0.007) [49]. Limited data are available on the safety of combining immunosuppressants with vedolizumab. However, based on available investigations, no combination therapy was shown to lead to an increase in adverse effects [50]. Moreover, combined therapy and thiopurine monotherapy resulted in a significantly higher proportion of patients with severe COVID-19 compared to TNF-antagonist monotherapy (8.8% and 9.2% vs. 2.2%, respectively, p < 0.001). The comparative analysis of TNF-antagonist monotherapy, combination therapy (adjusted OR 4.01, 95% CI 1.65–9.78), and thiopurine monotherapy (adjusted OR 4.08, 95% CI 1.73–9.61) showed a significantly increased risk of severe COVID-19 [51].
In summary, the combination of AZA with anti-TNF antibodies increases the effectiveness of the therapy. In patients who start therapy with IFX, combination therapy is recommended for about a year. During this treatment, doses of immunosuppressants should be lower than in monotherapy. In patients who discontinue biological treatment, it seems advisable to continue treatment with AZA. However, the potential risk of adverse effects should be assessed.
3.5. Thiopurine Cytotoxicity and Pregnancy in IBD
The safety of thiopurines in pregnancy has long been a controversial topic. There has long been evidence that both AZA, 6MP, and TG and their metabolites pass through the placenta to the fetus [52]. At the same time, a significant and positive correlation between infant and maternal 6TGN level at delivery was demonstrated. The last data including 40 pregnant IBD patients on thiopurines revealed that at delivery, the median 6TGN level was lower in infants than mothers in a ratio of 0.4:1 (78.5 vs. 217 pmol/8 × 108 RBCs, p < 0.001) [53].
The current state of knowledge shows that conventional thiopurine exposure throughout conception and pregnancy is considered safe and is not associated with a higher risk of preterm birth or congenital disorders [54][55]. Recently, Mahadevan et al., basing their analysis on prospective multicenter studies among 1490 completed pregnancies, demonstrated that thiopurines, anti-TNF drugs, or combination therapy during pregnancy were not associated with increased adverse maternal or fetal outcomes at birth or in the first year of life. Moreover, the data obtained confirm the impact of higher disease activity on adverse effects (spontaneous abortion, hazard ratio 3.41, 95% CI 1.51–7.69; and preterm birth with increased infant infection, OR 1.73, 95% CI 1.19–2.51) [56]. Therefore, clinical remission in IBD patients for at least a couple of months before conception and during pregnancy is significant to reduce the risk of spontaneous abortion and premature birth, and to promote reaching a healthy weight [57].
Nevertheless, in patients with IBD who are planning a pregnancy, particular attention should be paid to the level of metabolites of thiopurine drugs in the red blood cells (RBCs) and to the use of the available pharmacogenetic tools, for example, determination of TPMT and NUDT15 gene alleles [58].
3.6. Solutions to Cytotoxicity and Resistance to Thiopurines
In IBD patients, resistance to thiopurines and potent therapy cytotoxicity can be overcome by using a split dose of AZA or mercaptopurine ( for example, 50 mg twice a day instead of 100 mg once daily) calculated using the conventional weight-based dosing approach (AZA 2–2.5 mg/kg, 6MP 1–1.5 mg/kg). This solution was first described in 2012. On the one hand, it reduces 6-MMP metabolites and on the other, it maintains 6TGN levels, serving as an effective strategy to preserve immunomodulator therapy in IBD patients who have a preference for 6-MMP metabolism [43].
Another strategy is a combination of AZA or 6MP with allopurinol, an inhibitor of the XDH enzyme that saturates or reduces the TPMT methylation capacity (Figure 2). Several studies demonstrated a significant reduction in 6-MMP and an increase in the 6TGN level and clinical remission and mucosal healing of therapy in nearly half of IBD patients intolerant to conventional thiopurine therapy. However, numerous opportunistic infections occurred [59][60][61]. At present, the effects of allopurinol on the thiopurine metabolic pathway itself are still unknown. There are some hypotheses that this drug may damage HPRT or play a role in the methylation of thiopurines [62][63].
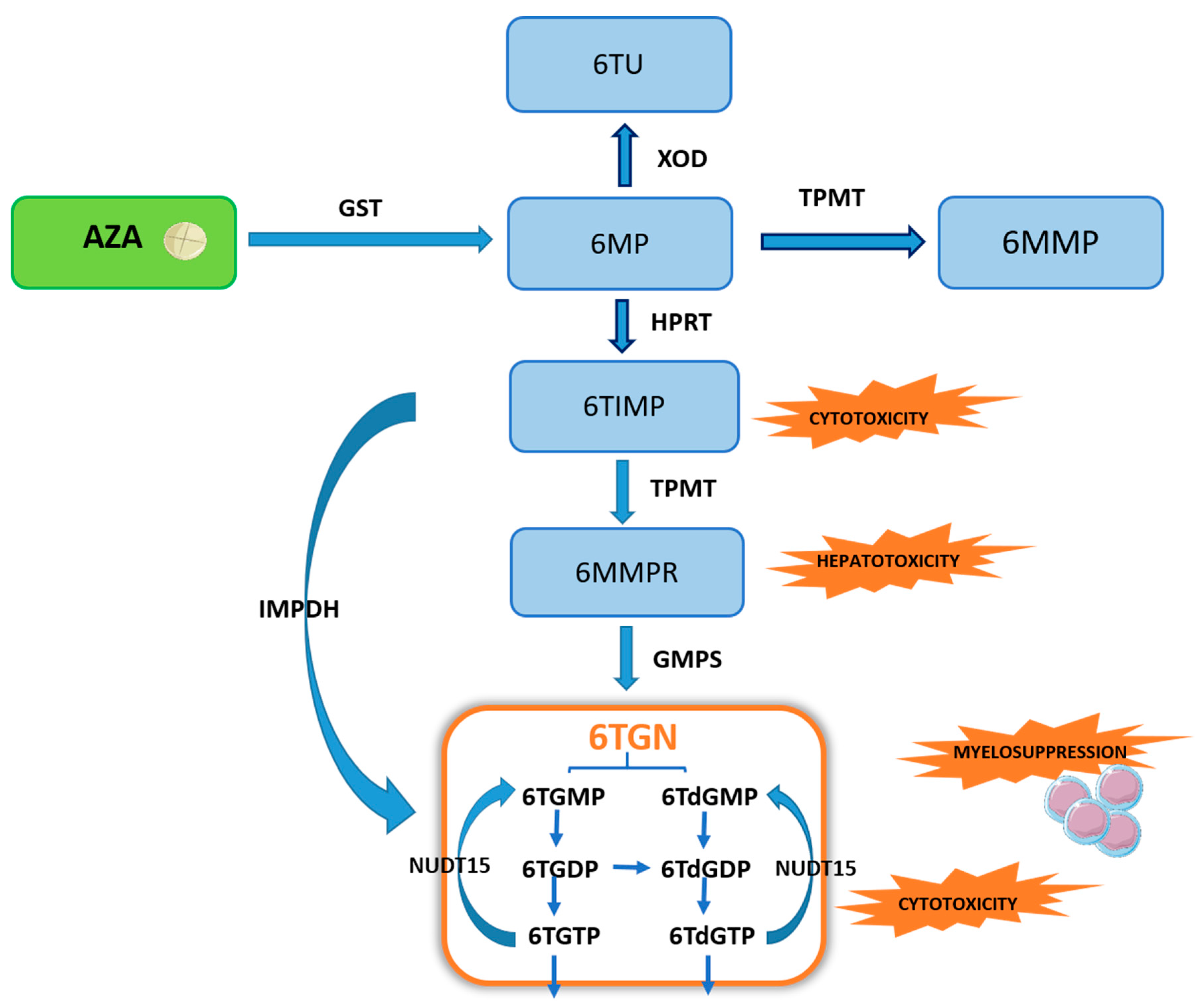
Figure 2. Scheme of the thiopurine biotransformation pathway. Explanation of abbreviations: AZA—azathioprine; GST—glutathione S-transferase; 6MP—6-mercaptopurine; XOD—xanthine oxidase/dehydrogenase; 6TUA—6-thiouric acid; TPMT—thiopurine methyltransferase; 6MMP—6-methylmercaptopurine; HPRT—hypoxanthine phosphoribosyltransferase; 6TIMP—6-thioinosine monophosphate; IMPDH—inosine monophosphate dehydrogenase; 6MMPR—6-methylmercaptopurine ribonucleotide; GMPS—guanosine monophosphate synthetase; 6TGN—6-thioguanine nucleotides; 6TGMP—6-thioguanosine monophosphate; 6TGDP—6-thio-guanosine diphosphate; 6TdGMP—6-thio-deoxyguanosine monophosphate; 6TdGDP—6-thiodeoxyguanosine diphosphate; 6TdGTP—6-thio-deoxyguanosine triphosphate; 6TGTP—6-thioguanosine triphosphate; NUDT15—nudix hydrolase motif 15.
In addition, a thiopurine alternative to common AZA and 6MP is also TG, which transformation pathway (to therapeutic TGN) is much shorter and has reduced cytotoxic potential. The conversion of TG to TGN requires only the participation of HGPRT, without ITPase, in contrast to the AZA and 6MP biotransformation (Figure 2 and Figure 3).
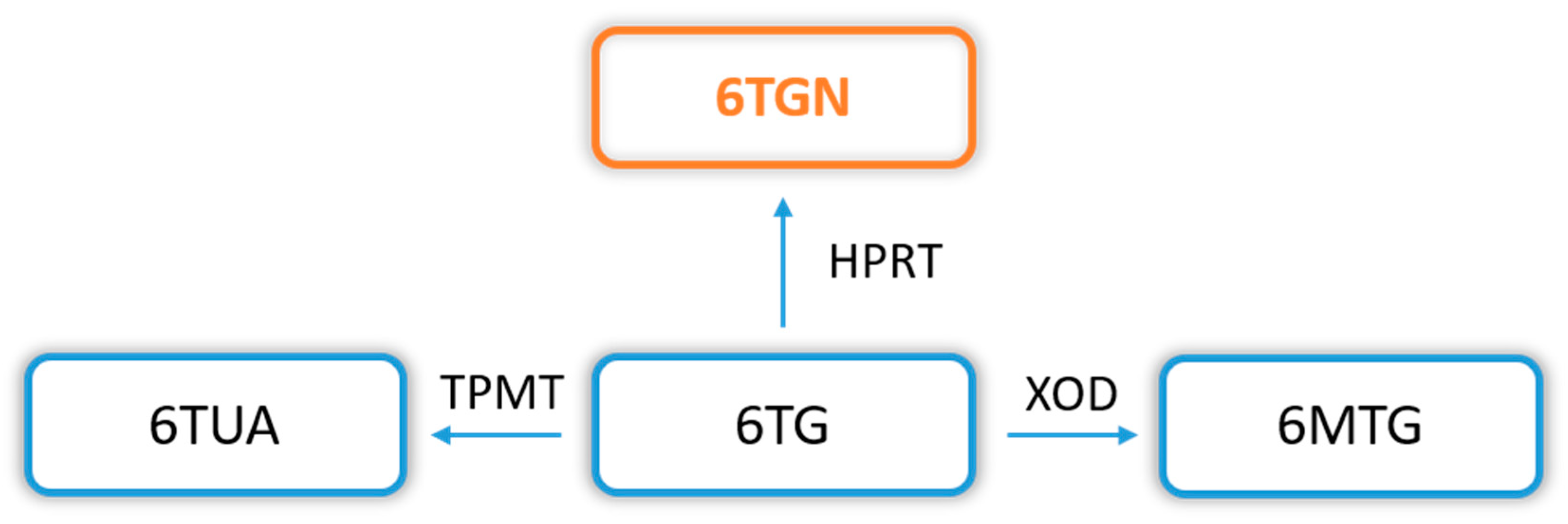
Figure 3. 6-thioguanine (6TG) biotransformation pathway. Explanation of abbreviations: 6TG—6-thioguanine; TPMT—thiopurine S-methyltransferase; 6TUA—6-thiouric acid; HPRT—hypoxanthine phosphoribosyltransferase; 6TGN—6-thioguanine nucleotides; XOD—xanthine oxidase/dehydrogenase; 6MTG—6-methyl thioguanine.
Treatment with 6TG is approved by the European Medicines Agency and the US Food and Drug Administration as an alternative to conventional thiopurine therapy in treating acute nonlymphocytic leukemia and acute lymphoblastic leukemia [64]. However, this therapeutic approach is not quoted in the IBD international guidelines. 6TG use has been restricted in IBD due to its association with the subsequent development of nodular regenerative hyperplasia of the liver and portal hypertension. However, this complication was observed in patients receiving high doses of 6TG, of up to 100 mg/day [65]. On the long-term safety of 6TG therapy in 274 IBD patients, who previously failed therapy with conventional thiopurines, demonstrated a therapeutic effect in 51%, and good toleration as a maintenance treatment for IBD in about 70% of patients [66]. In contrast to AZA and 6MP, the dose of 6TG does not depend on the patient’s weight and it amounts to that administered in a daily portion (20 mg/day). It was often indicated adverse events, but these were mainly mild or moderate. Therefore, 6TG in small doses is proposed as an effective therapy for IBD patients with a target threshold concentration of 6TGN ≥ 700 pmol/8 × 108 RBC [67].
This entry is adapted from the peer-reviewed paper 10.3390/toxics10040151
References
- Sahasranaman, S.; Howard, D.; Roy, S. Clinical Pharmacology and Pharmacogenetics of Thiopurines. Eur. J. Clin. Pharmacol. 2008, 64, 753–767.
- Amin, J.; Huang, B.; Yoon, J.; Shih, D.Q. Update 2014: Advances to Optimize 6-Mercaptopurine and Azathioprine to Reduce Toxicity and Improve Efficacy in the Management of IBD. Inflamm. Bowel Dis. 2015, 21, 445–452.
- Lucafò, M.; Stocco, G.; Martelossi, S.; Favretto, D.; Franca, R.; Malusà, N.; Lora, A.; Bramuzzo, M.; Naviglio, S.; Cecchin, E.; et al. Azathioprine Biotransformation in Young Patients with Inflammatory Bowel Disease: Contribution of Glutathione-S Transferase M1 and A1 Variants. Genes 2019, 10, 277.
- Stocco, G.; Cuzzoni, E.; De Iudicibus, S.; Franca, R.; Favretto, D.; Malusà, N.; Londero, M.; Cont, G.; Bartoli, F.; Martelossi, S.; et al. Deletion of Glutathione-S-Transferase M1 Reduces Azathioprine Metabolite Concentrations in Young Patients with Inflammatory Bowel Disease. J. Clin. Gastroenterol. 2014, 48, 43–51.
- Wong, D.R.; Coenen, M.J.H.; Derijks, L.J.J.; Vermeulen, S.H.; van Marrewijk, C.J.; Klungel, O.H.; Scheffer, H.; Franke, B.; Guchelaar, H.-J.; de Jong, D.J.; et al. The TOPIC Recruitment Team. Early Prediction of Thiopurine-Induced Hepatotoxicity in Inflammatory Bowel Disease. Aliment. Pharmacol. Ther. 2017, 45, 391–402.
- Dubinsky, M.C.; Lamothe, S.; Yang, H.Y.; Targan, S.R.; Sinnett, D.; Theoret, Y.; Seidman, E.G. Pharmacogenomics and Metabolite Measurement for 6-Mercaptopurine Therapy in Inflammatory Bowel Disease. Gastroenterology 2000, 118, 705–713.
- Cuffari, C. Thiopurine Methyltransferase Activity Influences Clinical Response to Azathioprine in Inflammatory Bowel Disease. Clin. Gastroenterol. Hepatol. 2004, 2, 410–417.
- Sayani, F.A.; Prosser, C.; Bailey, R.J.; Jacobs, P.; Fedorak, R.N. Thiopurine Methyltransferase Enzyme Activity Determination before Treatment of Inflammatory Bowel Disease with Azathioprine: Effect on Cost and Adverse Events. Can. J. Gastroenterol. 2005, 19, 147–151.
- Gisbert, J.P.; Niño, P.; Rodrigo, L.; Cara, C.; Guijarro, L.G. Thiopurine Methyltransferase (TPMT) Activity and Adverse Effects of Azathioprine in Inflammatory Bowel Disease: Long-Term Follow-Up Study of 394 Patients. Am. J. Gastroenterol. 2006, 101, 2769–2776.
- Derijks, L.J.J.; Gilissen, L.P.L.; Engels, L.G.J.B.; Bos, L.P.; Bus, P.J.; Lohman, J.J.H.M.; van Deventer, S.J.H.; Hommes, D.W.; Hooymans, P.M. Pharmacokinetics of 6-Thioguanine in Patients with Inflammatory Bowel Disease. Ther. Drug Monit. 2006, 28, 45–50.
- Haglund, S.; Vikingsson, S.; Söderman, J.; Hindorf, U.; Grännö, C.; Danelius, M.; Coulthard, S.; Peterson, C.; Almer, S. The Role of Inosine-5′-Monophosphate Dehydrogenase in Thiopurine Metabolism in Patients with Inflammatory Bowel Disease. Ther. Drug Monit. 2011, 33, 200–208.
- Kudo, M.; Moteki, T.; Sasaki, T.; Konno, Y.; Ujiie, S.; Onose, A.; Mizugaki, M.; Ishikawa, M.; Hiratsuka, M. Functional Characterization of Human Xanthine Oxidase Allelic Variants. Pharmacogenet. Genom. 2008, 18, 243–251.
- Wong, D.R.; Derijks, L.J.J.; den Dulk, M.O.; Gemmeke, E.H.K.M.; Hooymans, P.M. The Role of Xanthine Oxidase in Thiopurine Metabolism: A Case Report. Ther. Drug Monit. 2007, 29, 845–848.
- Valerie, N.C.K.; Hagenkort, A.; Page, B.D.G.; Masuyer, G.; Rehling, D.; Carter, M.; Bevc, L.; Herr, P.; Homan, E.; Sheppard, N.G.; et al. NUDT15 Hydrolyzes 6-Thio-DeoxyGTP to Mediate the Anticancer Efficacy of 6-Thioguanine. Cancer Res. 2016, 76, 5501–5511.
- Toyonaga, T.; Kobayashi, T.; Kuronuma, S.; Ueno, A.; Kiyohara, H.; Okabayashi, S.; Takeuchi, O.; Redfern, C.P.F.; Terai, H.; Ozaki, R.; et al. Increased DNA-Incorporated Thiopurine Metabolite as a Possible Mechanism for Leukocytopenia through Cell Apoptosis in Inflammatory Bowel Disease Patients with NUDT15 Mutation. J. Gastroenterol. 2021, 56, 999–1007.
- Tiede, I.; Fritz, G.; Strand, S.; Poppe, D.; Dvorsky, R.; Strand, D.; Lehr, H.A.; Wirtz, S.; Becker, C.; Atreya, R.; et al. CD28-Dependent Rac1 Activation Is the Molecular Target of Azathioprine in Primary Human CD4+ T Lymphocytes. J. Clin. Investig. 2003, 111, 1133–1145.
- Poppe, D.; Tiede, I.; Fritz, G.; Becker, C.; Bartsch, B.; Wirtz, S.; Strand, D.; Tanaka, S.; Galle, P.R.; Bustelo, X.R.; et al. Azathioprine Suppresses Ezrin-Radixin-Moesin-Dependent T Cell-APC Conjugation through Inhibition of Vav Guanosine Exchange Activity on Rac Proteins. J. Immunol. 2006, 176, 640–651.
- Daehn, I.; Brem, R.; Barkauskaite, E.; Karran, P. 6-Thioguanine Damages Mitochondrial DNA and Causes Mitochondrial Dysfunction in Human Cells. FEBS Lett. 2011, 585, 3941–3946.
- Warren, D.J.; Andersen, A.; Slørdal, L. Quantitation of 6-Thioguanine Residues in Peripheral Blood Leukocyte DNA Obtained from Patients Receiving 6-Mercaptopurine-Based Maintenance Therapy. Cancer Res. 1995, 55, 1670–1674.
- Rappaport, H.P. Replication of the Base Pair 6-Thioguanine/5-Methyl-2-Pyrimidinone with the Large Klenow Fragment of Escherichia Coli DNA Polymerase I. Biochemistry 1993, 32, 3047–3057.
- You, C.; Dai, X.; Yuan, B.; Wang, Y. Effects of 6-Thioguanine and S6-Methylthioguanine on Transcription in Vitro and in Human Cells. J. Biol. Chem. 2012, 287, 40915–40923.
- Yuan, B.; Zhang, J.; Wang, H.; Xiong, L.; Cai, Q.; Wang, T.; Jacobsen, S.; Pradhan, S.; Wang, Y. 6-Thioguanine Reactivates Epigenetically Silenced Genes in Acute Lymphoblastic Leukemia Cells by Facilitating Proteasome-Mediated Degradation of DNMT1. Cancer Res. 2011, 71, 1904–1911.
- Moriyama, T.; Nishii, R.; Perez-Andreu, V.; Yang, W.; Klussmann, F.A.; Zhao, X.; Lin, T.-N.; Hoshitsuki, K.; Nersting, J.; Kihira, K.; et al. NUDT15 Polymorphisms Alter Thiopurine Metabolism and Hematopoietic Toxicity. Nat. Genet. 2016, 48, 367–373.
- Coulthard, S.A.; Hogarth, L.A.; Little, M.; Matheson, E.C.; Redfern, C.P.F.; Minto, L.; Hall, A.G. The Effect of Thiopurine Methyltransferase Expression on Sensitivity to Thiopurine Drugs. Mol. Pharmacol. 2002, 62, 102–109.
- Coulthard, S.A.; Berry, P.; McGarrity, S.; Ansari, A.; Redfern, C.P.F. Liquid Chromatography–Mass Spectrometry for Measuring Deoxythioguanosine in DNA from Thiopurine-Treated Patients. J. Chromatogr. B 2016, 1028, 175–180.
- Torres, J.; Bonovas, S.; Doherty, G.; Kucharzik, T.; Gisbert, J.P.; Raine, T.; Adamina, M.; Armuzzi, A.; Bachmann, O.; Bager, P.; et al. ECCO Guidelines on Therapeutics in Crohn’s Disease: Medical Treatment. J. Crohns Colitis 2020, 14, 4–22.
- Harbord, M.; Eliakim, R.; Bettenworth, D.; Karmiris, K.; Katsanos, K.; Kopylov, U.; Kucharzik, T.; Molnár, T.; Raine, T.; Sebastian, S.; et al. Third European Evidence-Based Consensus on Diagnosis and Management of Ulcerative Colitis. Part 2: Current Management. J. Crohns Colitis 2017, 11, 769–784.
- Reinisch, W.; Panés, J.; Lémann, M.; Schreiber, S.; Feagan, B.; Schmidt, S.; Sturniolo, G.C.; Mikhailova, T.; Alexeeva, O.; Sanna, L.; et al. A Multicenter, Randomized, Double-Blind Trial of Everolimus Versus Azathioprine and Placebo to Maintain Steroid-Induced Remission in Patients with Moderate-to-Severe Active Crohn’s Disease. Am. J. Gastroenterol. 2008, 103, 2284–2292.
- Colombel, J.F.; Sandborn, W.J.; Reinisch, W.; Mantzaris, G.J.; Kornbluth, A.; Rachmilewitz, D.; Lichtiger, S.; D’Haens, G.; Diamond, R.H.; Broussard, D.L.; et al. Infliximab, Azathioprine, or Combination Therapy for Crohn’s Disease. N. Engl. J. Med. 2010, 362, 1383–1395.
- Jones, J.L.; Kaplan, G.G.; Peyrin-Biroulet, L.; Baidoo, L.; Devlin, S.; Melmed, G.Y.; Tanyingoh, D.; Raffals, L.; Irving, P.; Kozuch, P.; et al. Effects of Concomitant Immunomodulator Therapy on Efficacy and Safety of Anti–Tumor Necrosis Factor Therapy for Crohn’s Disease: A Meta-Analysis of Placebo-Controlled Trials. Clin. Gastroenterol. Hepatol. 2015, 13, 2233–2240.e2.
- Chande, N.; Patton, P.H.; Tsoulis, D.J.; Thomas, B.S.; MacDonald, J.K. Azathioprine or 6-Mercaptopurine for Maintenance of Remission in Crohn’s Disease. Cochrane Database Syst. Rev. 2015, 10, CD000067.
- Panés, J.; López–SanRomán, A.; Bermejo, F.; García–Sánchez, V.; Esteve, M.; Torres, Y.; Domènech, E.; Piqueras, M.; Gomez–García, M.; Gutiérrez, A.; et al. Early Azathioprine Therapy Is No More Effective Than Placebo for Newly Diagnosed Crohn’s Disease. Gastroenterology 2013, 145, 766–774.e1.
- Boyapati, R.K.; Torres, J.; Palmela, C.; Parker, C.E.; Silverberg, O.M.; Upadhyaya, S.D.; Nguyen, T.M.; Colombel, J.-F. withdrawal of Immunosuppressant or Biologic Therapy for Patients with Quiescent Crohn’s Disease. Cochrane Database Syst. Rev. 2018, 5, CD012540.
- Ardizzone, S. Randomised Controlled Trial of Azathioprine and 5-Aminosalicylic Acid for Treatment of Steroid Dependent Ulcerative Colitis. Gut 2006, 55, 47–53.
- Christophorou, D.; Funakoshi, N.; Duny, Y.; Valats, J.-C.; Bismuth, M.; Pineton De Chambrun, G.; Daures, J.-P.; Blanc, P. Systematic Review with Meta-Analysis: Infliximab and Immunosuppressant Therapy vs. Infliximab Alone for Active Ulcerative Colitis. Aliment. Pharmacol. Ther. 2015, 41, 603–612.
- Fraser, A.G. The Efficacy of Azathioprine for the Treatment of Inflammatory Bowel Disease: A 30 Year Review. Gut 2002, 50, 485–489.
- Privitera, G.; Pugliese, D.; Onali, S.; Petito, V.; Scaldaferri, F.; Gasbarrini, A.; Danese, S.; Armuzzi, A. Combination Therapy in Inflammatory Bowel Disease—From Traditional Immunosuppressors towards the New Paradigm of Dual Targeted Therapy. Autoimmun. Rev. 2021, 20, 102832.
- Brandse, J.F.; Mathôt, R.A.; van der Kleij, D.; Rispens, T.; Ashruf, Y.; Jansen, J.M.; Rietdijk, S.; Löwenberg, M.; Ponsioen, C.Y.; Singh, S.; et al. Pharmacokinetic Features and Presence of Antidrug Antibodies Associate with Response to Infliximab Induction Therapy in Patients with Moderate to Severe Ulcerative Colitis. Clin. Gastroenterol. Hepatol. 2016, 14, 251–258.e2.
- Rahier, J.F.; Magro, F.; Abreu, C.; Armuzzi, A.; Ben-Horin, S.; Chowers, Y.; Cottone, M.; de Ridder, L.; Doherty, G.; Ehehalt, R.; et al. Second European Evidence-Based Consensus on the Prevention, Diagnosis and Management of Opportunistic Infections in Inflammatory Bowel Disease. J. Crohns Colitis 2014, 8, 443–468.
- Chaparro, M.; Ordás, I.; Cabré, E.; Garcia-Sanchez, V.; Bastida, G.; Peñalva, M.; Gomollón, F.; García-Planella, E.; Merino, O.; Gutiérrez, A.; et al. Safety of Thiopurine Therapy in Inflammatory Bowel Disease: Long-Term Follow-up Study of 3931 Patients. Inflamm. Bowel Dis. 2013, 19, 1404–1410.
- Schwab, M.; Schaffeler, E.; Marx, C.; Fischer, C.; Lang, T.; Behrens, C.; Gregor, M.; Eichelbaum, M.; Zanger, U.; Kaskas, B. Azathioprine Therapy and Adverse Drug Reactions in Patients with Inflammatory Bowel Disease: Impact of Thiopurine S-Methyltransferase Polymorphism. Pharmacogenetics 2002, 12, 429–436.
- Lewis, J.D.; Abramson, O.; Pascua, M.; Liu, L.; Asakura, L.M.; Velayos, F.S.; Hutfless, S.M.; Alison, J.E.; Herrinton, L.J. Timing of Myelosuppression During Thiopurine Therapy for Inflammatory Bowel Disease: Implications for Monitoring Recommendations. Clin. Gastroenterol. Hepatol. 2009, 7, 1195–1201.
- Shih, D.Q.; Nguyen, M.; Zheng, L.; Ibanez, P.; Mei, L.; Kwan, L.Y.; Bradford, K.; Ting, C.; Targan, S.R.; Vasiliauskas, E.A. Split-Dose Administration of Thiopurine Drugs: A Novel and Effective Strategy for Managing Preferential 6-MMP Metabolism. Aliment. Pharmacol. Ther. 2012, 36, 449–458.
- Teich, N.; Mohl, W.; Bokemeyer, B.; Bündgens, B.; Büning, J.; Miehlke, S.; Hüppe, D.; Maaser, C.; Klugmann, T.; Kruis, W.; et al. Azathioprine-Induced Acute Pancreatitis in Patients with Inflammatory Bowel Diseases—A Prospective Study on Incidence and Severity. J. Crohns Colitis 2016, 10, 61–68.
- Ansari, A.; Arenas, M.; Greenfield, S.M.; Morris, D.; Lindsay, J.; Gilshenan, K.; Smith, M.; Lewis, C.; Marinaki, A.; Duley, J.; et al. Prospective Evaluation of the Pharmacogenetics of Azathioprine in the Treatment of Inflammatory Bowel Disease. Aliment. Pharmacol. Ther. 2008, 28, 973–983.
- Lichtenstein, G.R.; Rutgeerts, P.; Sandborn, W.J.; Sands, B.E.; Diamond, R.H.; Blank, M.; Montello, J.; Tang, L.; Cornillie, F.; Colombel, J.-F. A Pooled Analysis of Infections, Malignancy, and Mortality in Infliximab- and Immunomodulator-Treated Adult Patients with Inflammatory Bowel Disease. Am. J. Gastroenterol. 2012, 107, 1051–1063.
- Lichtenstein, G.R.; Feagan, B.G.; Cohen, R.D.; Salzberg, B.A.; Diamond, R.H.; Langholff, W.; Londhe, A.; Sandborn, W.J. Drug Therapies and the Risk of Malignancy in Crohn’s Disease: Results From the TREATTM Registry. Inflamm. BOWEL Dis. 2014, 109, 12.
- D’Haens, G.; Reinisch, W.; Panaccione, R.; Satsangi, J.; Petersson, J.; Bereswill, M.; Arikan, D.; Perotti, E.; Robinson, A.M.; Kalabic, J.; et al. Open: Lymphoma Risk and Overall Safety Profile of Adalimumab in Patients with Crohn’s Disease with up to 6 Years of Follow-up in the PYRAMID Registry. Am. J. Gastroenterol. 2018, 113, 872–882.
- D’Haens, G.; Reinisch, W.; Colombel, J.-F.; Panes, J.; Ghosh, S.; Prantera, C.; Lindgren, S.; Hommes, D.W.; Huang, Z.; Boice, J.; et al. Five-Year Safety Data From ENCORE, a European Observational Safety Registry for Adults with Crohn’s Disease Treated with Infliximab or Conventional Therapy. J. Crohns Colitis 2016, 11, 680–689.
- Feagan, B.G.; Rutgeerts, P.; Sands, B.E.; Hanauer, S.; Colombel, J.-F.; Sandborn, W.J.; Van Assche, G.; Axler, J.; Kim, H.-J.; Danese, S.; et al. Vedolizumab as Induction and Maintenance Therapy for Ulcerative Colitis. N. Engl. J. Med. 2013, 369, 699–710.
- Ungaro, R.C.; Brenner, E.J.; Gearry, R.B.; Kaplan, G.G.; Kissous-Hunt, M.; Lewis, J.D.; Ng, S.C.; Rahier, J.-F.; Reinisch, W.; Steinwurz, F.; et al. Effect of IBD Medications on COVID-19 Outcomes: Results from an International Registry. Gut 2021, 70, 725–732.
- De Boer, N.K.H.; Jarbandhan, S.V.A.; de Graaf, P.; Mulder, C.J.J.; van Elburg, R.M.; van Bodegraven, A.A. Azathioprine Use During Pregnancy: Unexpected Intrauterine Exposure to Metabolites. Am. J. Gastroenterol. 2006, 101, 1390–1392.
- Flanagan, E.; Wright, E.K.; Hardikar, W.; Sparrow, M.P.; Connell, W.R.; Kamm, M.A.; De Cruz, P.; Brown, S.J.; Thompson, A.; Greenway, A.; et al. Maternal Thiopurine Metabolism during Pregnancy in Inflammatory Bowel Disease and Clearance of Thiopurine Metabolites and Outcomes in Exposed Neonates. Aliment. Pharmacol. Ther. 2021, 53, 810–820.
- Kanis, S.L.; de Lima-Karagiannis, A.; de Boer, N.K.H.; van der Woude, C.J. Use of Thiopurines During Conception and Pregnancy Is Not Associated with Adverse Pregnancy Outcomes or Health of Infants at One Year in a Prospective Study. Clin. Gastroenterol. Hepatol. 2017, 15, 1232–1241.e1.
- Van der Woude, C.J.; Ardizzone, S.; Bengtson, M.B.; Fiorino, G.; Fraser, G.; Katsanos, K.; Kolacek, S.; Juillerat, P.; Mulders, A.G.M.G.J.; Pedersen, N.; et al. The Second European Evidenced-Based Consensus on Reproduction and Pregnancy in Inflammatory Bowel Disease. J. Crohns Colitis 2015, 9, 107–124.
- Mahadevan, U.; Long, M.D.; Kane, S.V.; Roy, A.; Dubinsky, M.C.; Sands, B.E.; Cohen, R.D.; Chambers, C.D.; Sandborn, W.J. Pregnancy and Neonatal Outcomes After Fetal Exposure to Biologics and Thiopurines Among Women with Inflammatory Bowel Disease. Gastroenterology 2021, 160, 1131–1139.
- Nguyen, G.C. The Toronto Consensus Statements for the Management of Inflammatory Bowel Disease in Pregnancy. Gastroenterology 2016, 150, 734–757.e1.
- Andoh, A.; Kawahara, M.; Imai, T.; Tatsumi, G.; Inatomi, O.; Kakuta, Y. Thiopurine Pharmacogenomics and Pregnancy in Inflammatory Bowel Disease. J. Gastroenterol. 2021, 56, 881–890.
- Govani, S.M.; Higgins, P.D.R. Combination of Thiopurines and Allopurinol: Adverse Events and Clinical Benefit in IBD. J. Crohns Colitis 2010, 4, 444–449.
- Hoentjen, F.; Seinen, M.L.; Hanauer, S.B.; de Boer, N.K.H.; Rubin, D.T.; Bouma, G.; Harrell, L.E.; van Bodegraven, A.A. Safety and Effectiveness of Long-Term Allopurinol–Thiopurine Maintenance Treatment in Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2013, 19, 363–369.
- Moreau, B.; Clement, P.; Theoret, Y.; Seidman, E.G. Allopurinol in Combination with Thiopurine Induces Mucosal Healing and Improves Clinical and Metabolic Outcomes in IBD. Ther. Adv. Gastroenterol. 2017, 10, 819–827.
- Seinen, M.L.; van Asseldonk, D.P.; de Boer, N.K.H.; Losekoot, N.; Smid, K.; Mulder, C.J.J.; Bouma, G.; Peters, G.J.; van Bodegraven, A.A. The Effect of Allopurinol and Low-Dose Thiopurine Combination Therapy on the Activity of Three Pivotal Thiopurine Metabolizing Enzymes: Results from a Prospective Pharmacological Study. J. Crohns Colitis 2013, 7, 812–819.
- Lim, S.Z.; Chua, E.W. Revisiting the Role of Thiopurines in Inflammatory Bowel Disease Through Pharmacogenomics and Use of Novel Methods for Therapeutic Drug Monitoring. Front. Pharmacol. 2018, 9, 1107.
- Crouwel, F.; Simsek, M.; Mulder, C.J.J.; Buiter, H.J.C.; De Boer, N.K. Thioguanine Therapy in Inflammatory Bowel Diseases. A Practical Guide. J. Gastrointestin. Liver Dis. 2020, 29, 637–645.
- Dubinsky, M.C.; Vasiliauskas, E.A.; Singh, H.; Abreu, M.T.; Papadakis, K.A.; Tran, T.; Martin, P.; Vierling, J.M.; Geller, S.A.; Targan, S.R.; et al. 6-Thioguanine Can Cause Serious Liver Injury in Inflammatory Bowel Disease Patients. Gastroenterology 2003, 125, 298–303.
- Biemans, V.B.C.; Savelkoul, E.; Gabriëls, R.Y.; Simsek, M.; Dijkstra, G.; Pierik, M.J.; West, R.L.; de Boer, N.K.H.; Hoentjen, F. A Comparative Analysis of Tioguanine versus Low-Dose Thiopurines Combined with Allopurinol in Inflammatory Bowel Disease Patients. Aliment. Pharmacol. Ther. 2020, 51, 1076–1086.
- Simsek, M.; Deben, D.S.; Horjus, C.S.; Bénard, M.V.; Lissenberg-Witte, B.I.; Buiter, H.J.C.; van Luin, M.; Seinen, M.L.; Mulder, C.J.J.; Wong, D.R.; et al. Sustained Effectiveness, Safety and Therapeutic Drug Monitoring of Tioguanine in a Cohort of 274 IBD Patients Intolerant for Conventional Therapies. Aliment. Pharmacol. Ther. 2019, 50, 54–65.
This entry is offline, you can click here to edit this entry!
