Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
As a result of the COVID-19 pandemic, various joint efforts have been made to support the creation of vaccines. Different projects have been under development, of which some are in the clinical evaluation stage and others in are in phase III with positive results.
- SARS-CoV-2
- COVID-19
- vaccines
- immunity
- efficacy
1. Introduction
Until recently, the coronavirus family was not considered highly contagious. Human coronaviruses, such as HCoV-229E, HCoV-OC43, HCoV-NL63, and HCoV-HKU1, are very common and usually cause colds like other pathogens but have low immunogenicity [1]. However, since SARS-CoV appeared in 2002–2003 and MERS-CoV in 2012, health authorities worldwide began to pay attention to the behavior and dispersal of these genotypes. As a result, various research lines emerged to understand better their pathogenicity and immunogenic capacity [2].
At the end of 2019, a new disease was announced caused by a viral agent belonging to the coronavirus family. It was named SARS-CoV-2 due to its relationship with the virus that causes Severe Acute Respiratory Syndrome (SARS) and the infection mechanism is relies on the protein “S”, which is composed of two subunits, S1, which contains the receptor-binding domains that recognizes and binds to the host receptor angiotensin-converting enzyme 2 (ACE-2), and subunit S2, which mediates viral cell membrane fusion [3]. Its infection causes the disease COVID-19, an acronym for coronavirus disease with the year it was discovered. The first registered case occurred in Wuhan, China, on 31 December 2019 [4].
2. COVID-19 Vaccines Development
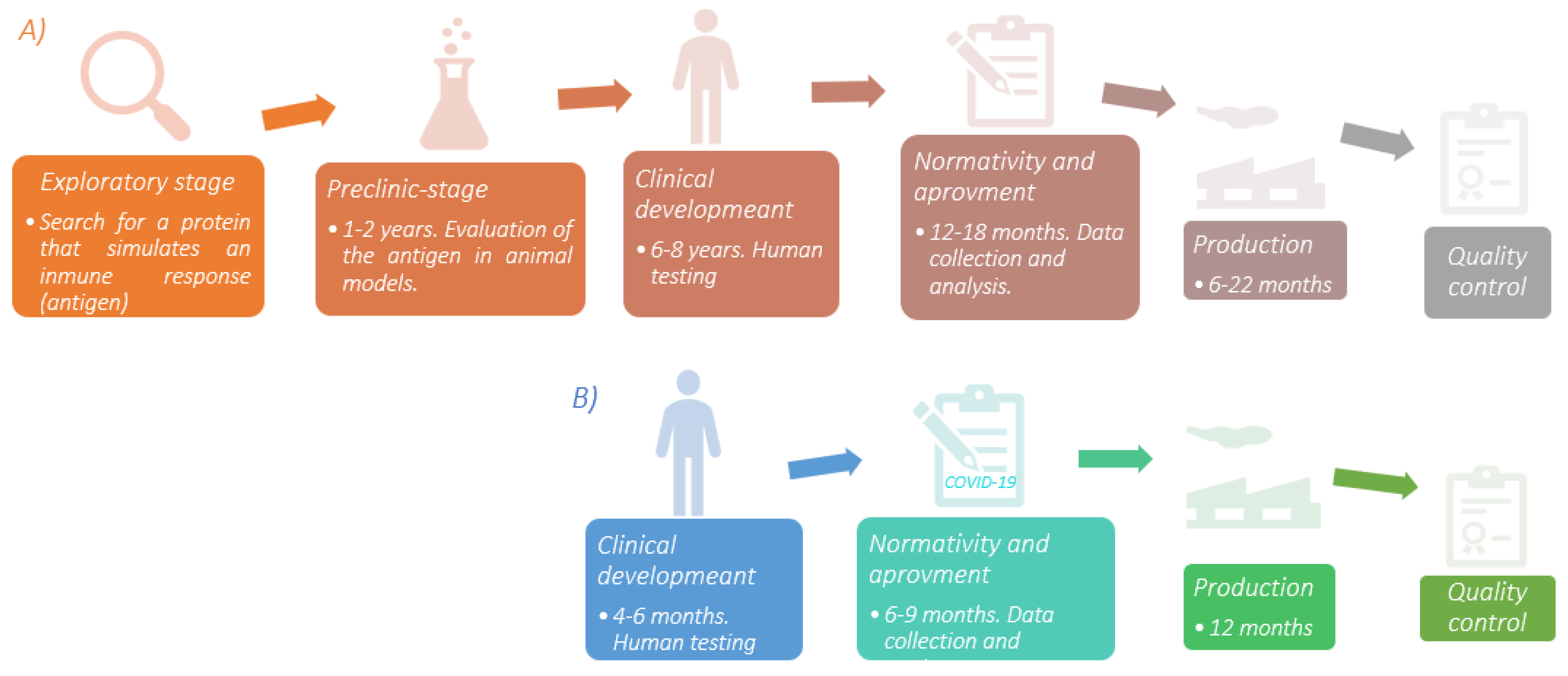
Developing a vaccine is a complex process that can take up to 8 years before being available to the population. In addition, strict protocols must be followed to guarantee its safety and efficacy. However, for COVID-19, it was completely different. There is a 79.5% genetic similarity between SARS-CoV and SARS-CoV-2 [5]. Thus, the previous knowledge gathered from the first investigations on this virus family that began almost two decades ago proved to be relevant for developing vaccines against SARS-CoV-2 and, of course, producing them in record time. Additionally, the contributions of Katalin Karikó regarding the use of modified mRNA as a tool for immunological purposes [6] allowed the global pharmaceutical industry to start producing these vaccines. A brief scheme of the entire vaccine development process and COVID-19 vaccine is shown in Figure 1.
The development of the different types of vaccines shows the immense technological advances that exist today (Table 1). However, like any tool, they have advantages and disadvantages (Table 2) that must be pondered before choosing the most appropriate one. Moreover, vaccines can also enhance the immune response with the help of substances called adjuvants.
Table 1. Platforms used for the development and production of vaccines.
| Type of Vaccine | Development | Invention Year | Target Desease |
|---|---|---|---|
| Attenuated pathogen | Through physicochemical treatments, the pathogen loses features that allow an effective infection. Due to the intact antigens on the membrane surface, they can be recognized by the immune system. | 1798 | Smallpox |
| Dead/inactivated pathogen | Through physicochemical treatments, the bacterial pathogen is killed and viral pathogen is inactivated. It cannot infect, but the antigens must remain on the membrane to be recognized by the immune system. | 1896 | Typhoid |
| Toxoids | The bacterial toxins are attenuated with chemical agents such as formaldehyde or the effects of heat, preserving their high immunogenicity. | 1923 | Diphtheria |
| Protein subunits | They contain only harmless proteins of the microorganism. They are made by recombinant expression in cell models such as bacteria or fungi, or obtained by lysis of the pathogen, but the proteins that join to the host’s receptors are preserved to protect their three-dimensional conformation. | 1970 | Anthrax |
| Viral particles | The structural proteins of the pathogen are assembled using a matrix (which can be a lipid bilayer) which allows simulating the pathogen’s spatial conformation without genetic content. They have high immunogenicity since most of the pathogen’s proteins are present. | 1986 | Hepatitis B |
| Viral Vectors | These are genetically modified viruses, which have already been well characterized. The genetic content is eliminated, except for those genes that give a cell the ability to infect. The removed genetic material is replaced by that that is of interest (DNA or mRNA *) and incorporated into the virus for protection and transport. Once the vector comes into contact with human cells, it instructs them to produce a protein exclusive for the microorganism. Thus, the body begins to manufacture components of the immune system. Most of these viral vectors cannot replicate. | 2019 | Ebola |
| Nucleic acids | They can be DNA or mRNA. In both cases, the genetic material is protected by a nanoparticle, mainly lipids, since it becomes permeable to the phospholipid bilayer of the cell membrane. DNA travels through the cytosol until incorporated into the nucleus, where it is transcribed into mRNA and later translated into a chain of amino acids. Something similar happens with the mRNA, but it does not enter the nucleus, instead passsing directly to the ribosomes to synthesize the chain of amino acids. Finally, this genetic material allows the production of pathogenic proteins which will be expressed at the membrane surface level, thus achieving the creation of antigens through our cells, which will stimulate the immune system. | 2020 | SARS-CoV-2 |
Table 2. Advantages and disadvantages of different platforms for vaccine development.
| Platform | Advantages | Disadvantages |
|---|---|---|
| Attenuated pathogen | Produces humoral and cellular response with a single dose. | Safety problems in immunosuppressed people. Strains are difficult to obtain. |
| Dead/inactivated pathogen |
Safe due to the nature of its composition. Very easy to transport and store. |
Large amounts of the pathogen. Possible effects on the immunogenicity of the antigen |
| Protein subunits | Safe during production and for immunosuppressed people. | Decrease in APC * capacity due to particle size. Limited production due to product scalability. |
| Polysaccharides | Alternative against bacterias with abundant polysaccharide antigens. | There is only IgM production. Low memory immunity. Low efficiency in children. |
| Viral particles | Combines the efficacy of live and subunit vaccines. High scalability production. |
Particle assembly is a complex process. |
| Viral Vectors | It can induce a humoral and cellular response. Safe. |
Pre-existing immunity is used against the vector. It needs low temperatures to store. |
| Nucleic acid | Scalability. Rapid design and development. Very secure. Induces humoral and cellular responses. |
Its storage and handling are delicate. |
Figure 1. (A) Sequential diagram of the vaccine development process. The exploratory stage is the basis for the evolution of the product under development; finding an antigen that correctly stimulates the immune system allows the rest of the process to continue. Human testing is the most time-consuming stage due to the number of individuals involved in the study. In addition, a wide range of regions must be included to assess environmental and genetic conditions and detect if there are differences between study groups. (B) COVID-19 vaccine development is one of the greatest accomplishments in medical history. Due to the vast contribution of scientific research in SARS-CoV, the production of this vaccine was faster compared to others. The time described is according to the clinical trials for the different vaccines. Source: [11][12][13] Design: Carlos U. Torres-Estrella.
Adjuvants
Aluminum salts as adjuvants have been used in different vaccines, such as the PiCoVacc and CoV-RBD219N1 vaccines for the SARS-CoV-2 virus, promoting higher antibody production [13]. The Novavax vaccine uses Matrix-M1 as an adjuvant, based on purified saponin obtained from the Quillaja saponaria tree, formulated with cholesterol nanoparticles and phospholipids [14]. This adjuvant enhances the immune response by recruiting a larger amount of APC at the application site, which increases the presentation of antigens to T cells, generating a TH1 and TH2 response [15]. In addition, it induces antibodies of multiple subclasses, mainly high titers of anti-S IgG that block the binding of the ACE2 receptor, neutralizing the virus, reducing the dose of antigen used in each vaccine, and reducing costs [16]. SARS-CoV-2 vaccines formulated with soluble homogeneous “Spike” protein S trimers (S-2P) use AS03 as an adjuvant, an oil-in-water emulsion composed of squalene, polysorbate 80, and α-tocopherol. This compound induces an IgG antibody response sufficient to protect from exposure to SARS-CoV-2 [17]. The Sclamp vaccine, which is in the preclinical stage, consists of stabilized recombinant viral “Spike” glycoprotein S in its trimeric form used in combination with the MF59 adjuvant, which consists of a 4.3% squalene oil emulsion in water, stabilized with Tween 80 and Span 85. This adjuvant interacts with the APCs at the inoculation site and participates in the transfer of antigens, increasing the efficiency of antigen presentation and stimulating a significant immune response mediated by neutralizing antibodies [18]. The CIGB2020 compound, produced by the Center for Genetic Engineering and Biotechnology (CIGB) in Havana, Cuba, has been used to formulate vaccines against hepatitis. It stimulates specific genes to produce interferons and other cytokines with antiviral properties, which also intervene in the expression of class I and II human leukocyte antigen (HLA) molecules. These molecules are involved in the activation of the innate immune system and increase the presentation of viral antigens, and therefore, stimulate T cells [19]. In March 2020, clinical trials were initiated to explore and evaluate the immunotherapeutic effect of this substance in suspected and confirmed patients with SARS-CoV-2 infection. However, the results of this protocol have not been published yet [20].
3. COVID-19 Vaccines
The available information about this coronavirus family was immensely advantageous compared to other cases since, as explained in Figure 1, the time required to formulate a vaccine is considerable. Table 3 and Table 4 list the main vaccines currently available and those used since the beginning of 2021 in the global vaccination campaign to counteract the number of infections and victims of COVID-19 disease.
Table 3. Primary nucleic acid-based vaccines and viral vectors available for use in some regions of the world.
| Type of Vaccine | |||||||
|---|---|---|---|---|---|---|---|
| Nucleic Acids | Vector Viral | ||||||
| Name | Comirnaty (BNT162b2 mRNA) | mRNA-1273 | CVnCoV | AZD1222 (ChAdOx1) |
Ad5-nCov | Sputnik V (Gam-COVID-Vac) | Ad26.COV2.21S (JNJ-78436735 |
| Manufacturing Company | Pfizer/BioNTech | MODERNA | CureVac/Bayer/GSK/No vartis | AstraZeneca/Oxford | CanSino Biological | Gamaleya Research Institute | Janssen Pharmaceutical Companies of Johnson & Johnson (J & J) |
| Handling/ Storage |
−70 °C up to 6 months, 2–8 °C up to 5 days, reconstituted up to 6 hrs | −20 °C up to 6 months, 2–8 °C up to 30 days | 2–8 °C up to 3 months | 2–8 °C | 2–8 °C | 1st vial frozen at −18 °C 2nd vial lyophilized at 2–8 °C |
2–8 °C |
| Doses required | Three doses Second dose 21–42 days after the first dose Third dose 6 to 12 months after the second dose |
Second dose 28 days after the first one | Second dose 28 days after the first one | Second dose 28 days after the first one | Single dose | Second dose 21 days after the first one | Single dose * |
| Immunization per dose | 100 µg 30 µg (3rd doses) |
30 µg | 12 µg | 0.5 × 1011 Vp | 0.5 × 1011 Vp | 0.5 mL | 0.5 × 1011 Vp |
| % Efficacy in preventing infection | 95% | 94.1% | Phase III data to be published | 82.4% | 65.28% | 92% | 72% in the USA 61% in Latin America |
| Observations | It contains a strand of mRNA that codes for the protein S “Spike” wrapped in a lipid nanoparticle using polyethylene glycol as a stabilizing agent. The third dose is being evaluated in patients 18–55 years and 65–85 years. | It contains a strand of mRNA that codes for the protein S “Spike” wrapped in a lipid nanoparticle. | It contains a strand of mRNA that codes for the protein S “Spike” wrapped in a lipid nanoparticle. Mexico is one of the countries selected for phase III. | Chimpanzee adenovirus containing mRNA encoding protein S “Spike.” | Modified adenovirus serotype Ad5 containing mRNA encoding protein S “Spike.” | The first vial is a modified adenovirus serotype Ad26. The second one is a modified adenovirus serotype Ad5. Both contain double-stranded DNA with the S gene for the “Spike” protein. | Modified adenovirus serotype Ad26 containing double-stranded DNA with the “Spike” protein S gene. |
EMA: European Medicines Agency; Vp: Viral particles; mRNA: Ribonucleic acid of the messenger type; DNA: Deoxyribonucleic Acid; * J & J Pharmaceuticals indicates that two doses may be required depending on the patient’s needs and the health care provider’s determination. They all contain the “S” gene in the form of mRNA or DNA [21][22][23][24][25][26][27][28][29][30][31][32].
Table 4. Primary vaccines based on the attenuated SARS-CoV-2 virus and protein “S” subunits available for use in some regions of the world.
| Type of Vaccine | ||||||
|---|---|---|---|---|---|---|
| Characteristics | Attenuated Pathogen | Protein Subunities | ||||
| Name | CoronaVac | Covaxin (BBV152 A, B, C) | Not Available | BBIBP-CorV | NVX-CoV2373 | ZF2001 |
| Manufacturing Company/Institution | Sinovac | Bharat Biotech/Indian Council of Medical Research | Sinopharm/Wuhan Institute of Biological Products | Sinopharm/Beijing Institute of Biological Products | NOVAVAX | Anhui Zhifei Longcom Biopharmaceutical Co./Government of Uzbekistan |
| Handling/Storage | 2–8 °C | 2–8 °C | 2–8 °C | 2–8 °C | 2–8 °C | 2–8 °C |
| Doses required | Second dose 14 days after the first one | Second doses 28 days after the first one | Second dose 21 days after the first one | Second dose 21 days after the first one | Second dose 21 days after the first one | 2–3 doses 28 days after the first one |
| Immunization per dose | 3 µg | 3 µg | Unknown | 4 µg | 5 µg SARS-CoV-2 rS + 50 µg of Matrix-M1 adjuvant | 25 µg/0.5 mL |
| % Efficacy in preventing infection | 83.7% in Turkey 50.3% in Brazil |
81% | 72.5% | 79.34% | 96% Original coronavirus 86% variant B.1.1.7 49% variant B.1.351 |
Not reported |
| Observations | - | - | - | - | Nanoparticles containing the protein subunit S. | Recombinant origin using CHO cell line to express protein S. |
CHO: Chinese Hamster Ovary; Adjuvant: A molecule that helps increase the immune response; B.1.1.7: British variant; B.1.351: South African variant [16][24][33][34][35][36].
Most of these vaccines have been approved by the WHO with an “emergency” use license; that is, these are vaccines that present conclusive data regarding the level of conferred protection, safety, and efficacy when monitoring the population selected for the clinical trials for at least two months [37]. However, on 23 August 2021, and 31 January 2022, the FDA approved with a full license the use of Pfizer/BioNTech and MODERNA vaccines, respectively [38][39]. It also must be considered that public and private investments, which are unprecedented events, allowed the great researchers of the 21st century to act immediately to develop these vaccines. Unfortunately, some of these products have encountered problems during application in various regions. The first complication occurred with the Pfizer/BioNTech vaccine. A case was reported of a person who developed an exacerbated allergy to one of the primary vaccine compounds, polyethylene glycol, an allergenic molecule for certain people. Therefore, it is recommended to conduct a comprehensive review of the patients’ medical history to detect any known allergies [22]. On the other hand, after receiving the Astra Zeneca/Oxford vaccine, the presence of blood clots that caused either thrombosis or thrombocytopenia was reported in certain patients. This incident led some countries to suspend vaccination with this vaccine, even though 20 million doses had already been applied. Shortly after, on 19 March 2021, the WHO made a public statement in conjunction with the Pharmacovigilance Risk Assessment Committee (PRAC) of the European Medicines Agency (EMA) regarding these events. The statement read that all adverse events had been carefully analyzed and that pondering the number of vaccine doses already applied, the number of reported cases was not relevant to conclude that the vaccine was unsafe. Additionally, the number of reports was within the permissible limits for these very rare events. Finally, they stressed that the benefits provided by the vaccine were more significant than the possible side effects that may arise and that it is essential to assess the clinical conditions of each patient for these events [40].
Still, many other projects are in the early stages despite financing issues and specialized human resources needs. These enterprises will undoubtedly promise a very competitive market, in which purchasing power will not be a problem for less developed countries as there will be an extensive manufacturer catalog [41].
3.1. Dose Immunization
Viral vector and nucleic acid vaccines require the application of two doses. The reason why this vaccination scheme is needed is that the immune system works in two ways. The first time there is contact with a pathogen, nonspecific IgM-type antibodies are produced (Figure 2A) that will help to block the pathogen during the primary response, which begins within three to five days. Then, highly specific and low-molecular-weight IgG-type antibodies (Figure 2B) are produced 10 to 15 days later. This adaptive response can last weeks and even months [42] (Figure 3). Because it is a parenteral type of immunity, the production of these immunoglobulins in the bloodstream is stimulated to avoid possible damage at the systemic level [43], along with a small synthesis of IgA immunoglobulins.
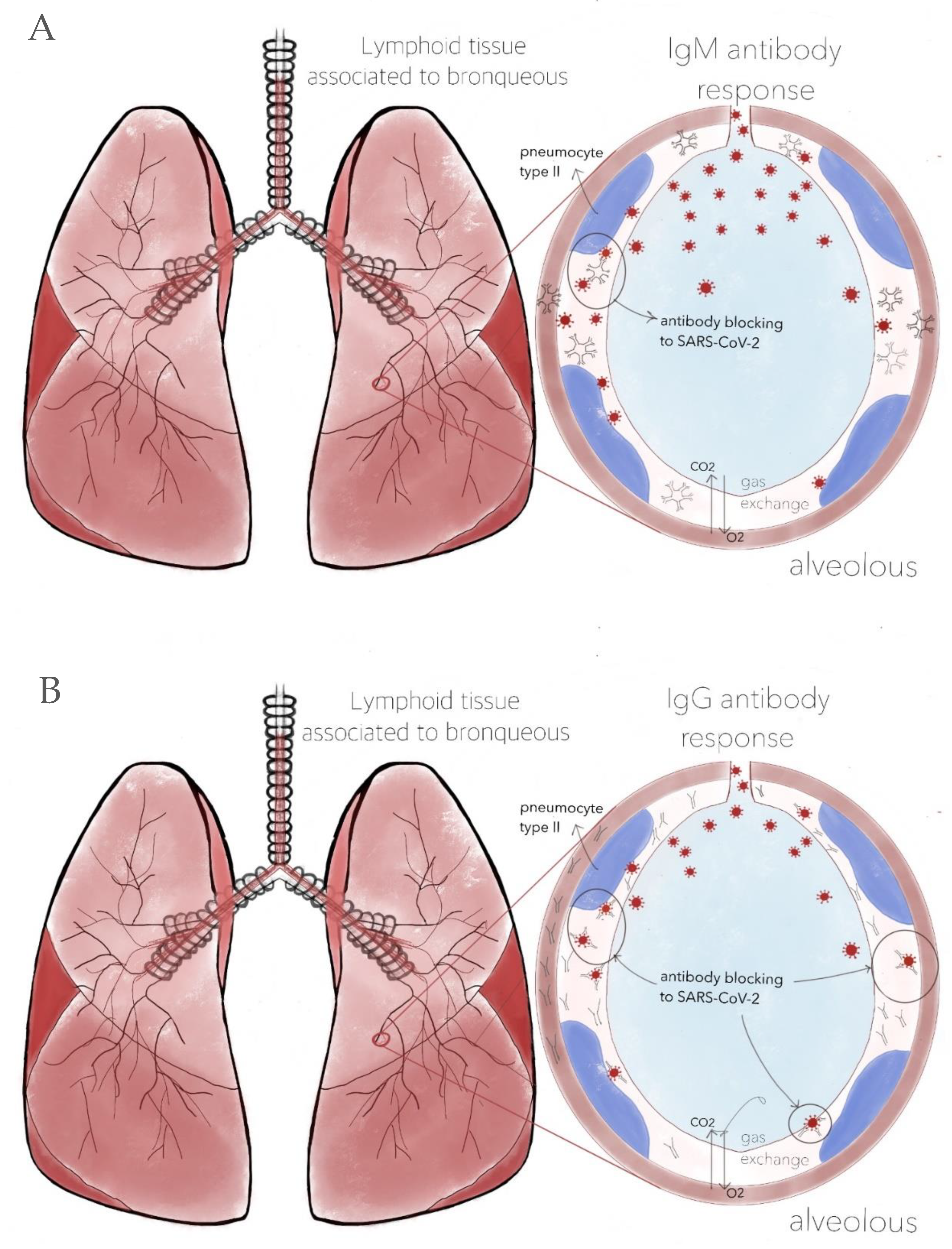
Figure 2. (A) Production of IgM antibodies after the first dose. Although there is a considerable quantity of immunoglobulins, their ability to neutralize SARS-CoV-2 is limited due to their size. Additionally, being very heavy, they cannot easily cross the capillary alveolus barrier due to the significant variability in their antigen-binding fragments (Fab). (B) IgM and IgG antibodies coexist. The latter are more specific and capable of neutralizing SARS-CoV-2. Due to their lower molecular weight, they manage to cross the capillary alveolus barrier. However, this does not mean that infection cannot occur; it only decreases the risk of developing COVID-19. Therefore, it is necessary to continue with adequate sanitary measures. Design by Carlos U. Torres-Estrella.
The genetic material contained in the vaccine is limited to the number of copies supplied to the person receiving it. Not all will translate into a protein, as some will degrade due to vesicular trafficking. Thus, a second dose ensures that the adaptive response is activated more efficiently and that the number of translated proteins is enough for a response. However, this is not strong enough to keep immune levels up. Due to the fact that the immune response decreases, a third dose is necessary to maintain the protection (Figure 3) according to the Center for Disease Control and Prevention (CDC) and the Johns Hopkins medical school [44][45].
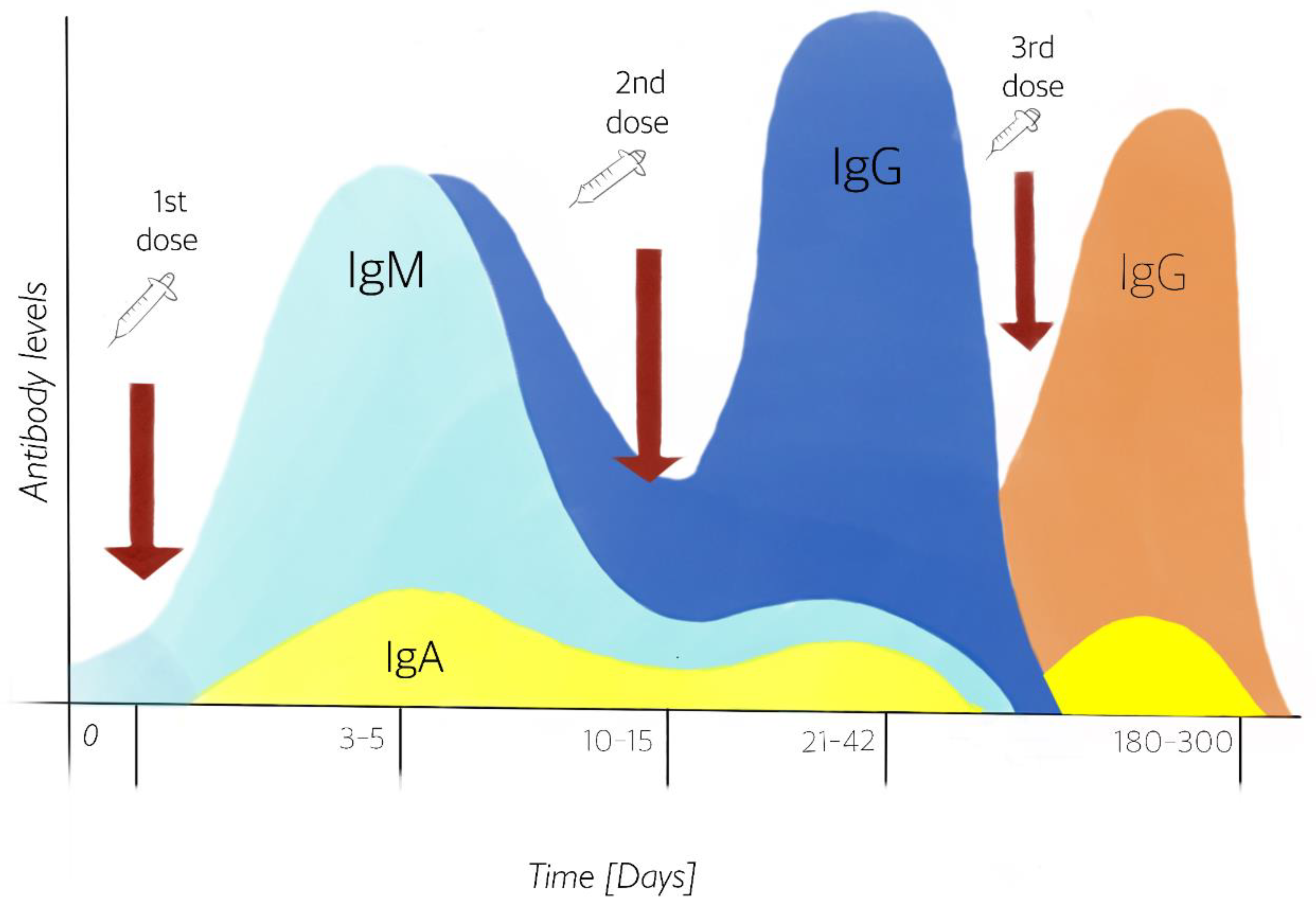

Figure 3. Production of IgM and IgG in serum, and IgA in mucosal surface antibodies from the first and second doses, simulating a first exposure to the pathogen and its immune memory. The stimulation of antibody IgA has been demonstrated after 1st dose vaccination [46]. Third dose guarantees to remain protected after six months. Design by: Carlos U. Torres-Estrella.
3.2. Heterologous Vaccines
It is known that booster immunization with heterologous vaccines can increase the intensity and amplitude of immune responses. Therefore, in the face of the current pandemic caused by the SARS-CoV-2 virus, heterologous vaccines against COVID-19 have emerged as a valuable alternative to provide the necessary immunological protection to the affected population. He et al. [47] conducted a study aimed at maximizing the benefits of vaccination using a heterologous booster strategy in a murine model, applying different combinations of four types of leading candidate vaccines against COVID-19 that were found in clinical trials in China. Their results showed that sequential immunization with an adenovirus-vectored vaccine followed by administering an inactivated/recombinant subunit/mRNA vaccine specifically increased neutralizing antibody levels and promoted the modulation of immune response antibodies to predominantly neutralizing antibodies. In addition, the heterologous priming and boosting regimen with an adenovirus vector-based vaccine also enhanced Th1-biased T cell responses. Thus, their results provided new ideas for developing and applying COVID-19 vaccines to control the SARS-CoV-2 pandemic.
Likewise, a study conducted by Hillus et al. [48], evaluated the reactogenicity and immunogenicity of heterologous immunizations with ChAdOx1 nCov-19 (AstraZeneca, Cambridge, UK) and BNT162b2 (Pfizer-BioNTech, Mainz, Germany), in comparison with homologous immunization with BNT162b2 and ChAdOx1 nCov-19. The study showed evidence of the safety and immunogenicity of heterologous ChAdOx1 nCov-19-BNT162b2 vaccination, currently recommended in several countries. Thus, their data support ongoing efforts to investigate heterologous vaccination regimens for COVID-19.
On the other hand, Eroglu et al. [49] mentioned that the preliminary results of a trial with CombiVacS that included more than 600 patients in Spain showed the benefits of mixing different vaccines against coronavirus. In addition, they mentioned that the combination of the Oxford–AstraZeneca vaccine and the Pfizer–BioNTech vaccine also induced a stronger immune response than two doses of the same vaccine. However, they emphasized that it is essential to consider that some safety concerns remain when two different vaccines are combined as each vaccine has its own set of adverse events/side effects. Nevertheless, no severe side effects were reported in the combined trials.
This entry is adapted from the peer-reviewed paper 10.3390/vaccines10030414
This entry is offline, you can click here to edit this entry!