Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Engineering, Biomedical
Infusion-based drug delivery, which directly administer to local tissue under a positive pressure gradient, as in convection-enhanced delivery (CED), provides an important opportunity to overcome the blood-brain barrier and dependency on type of drug. However, poor understanding of the pressure-driven drug transport mechanisms in the brain has hindered ultimate success of CED-like technologies in clinical applications.
- convection-enhanced delivery
- brain
- infusion
- fluid flow
- mass transport
1. Introduction
Tumours in the central nervous system (CNS) are some of the most prevalent, lethal and yet poorly treated diseases within the brain. Glioblastoma multiforme (GBM), a grade IV glioma, is the fastest-growing and most aggressive malignant primary CNS tumour in adults. It primarily occurs in older patients, with an average age of 64 years at diagnosis. Survival rates are poor, with approximately 40% survival in the first year post diagnosis and 17% in the second year. GBMs lead to about 250,000 deaths per year worldwide, and their treatment cost in Europe in 2010 was about 5.2 billion euros [1][2][3]. Conventional techniques such as chemotherapy and radiation are not effective in treating GBM. They either suffer from limitations in passing drugs through the blood–brain barrier (BBB) or unwanted drug distribution throughout the tissue due to passive diffusion and poor delivery to the target, or they are not viable because of severe side effects, e.g., localised tissue damage [4][5][6]. The drug effects within the CNS are driven by the concentration–time profile at the target site, and therefore, drugs need to reach the target for as long as needed and in an appropriate concentration, neither of which is easily achievable with conventional diffusion-based delivery methods. To overcome these challenges, an emerging approach is infusion-based targeted drug delivery, such as convection-enhanced delivery (CED), performed with robotic steerable needles [7][8].
Advancements in medical robotics through technical innovations has led to significant improvements in CED-like technologies [9][10]; however, ultimate success in the clinical applications of these systems remains a goal to be accomplished [7]. Current embodiments suffer from a lack of precise information and reliable experimental data on the flow behaviour in the brain, which limits the development of precise numerical models and their implementation in automated surgical systems. In fact, to progress towards a reliable and automated system for infusion-based targeted drug delivery to the brain, advancements on four fronts are to be made: (1) characterisation and understanding of drug flow behaviour in complex brain tissue, (2) developing realistic models based on experimental data that can predict drug infusion at the pre-operative stage, (3) technical innovation in drug delivery tools, and (4) their clinical deployment in Randomised Controlled Trials (RCTs).
2. Brain Tissue: A Complex System for Diffusion-Based Drug Delivery
The brain is a biological system mainly composed of neurons and neuroglia and possesses extreme complexity arising from the interaction of about 86 billion neurons and 100 trillion connections [11]. A prominent obstacle to the drug transport inside the brain is the BBB, which separates blood from the brain. The BBB, primarily formed by brain capillary endothelial cells connected by tight junctions that constitute the walls of the brain capillaries, is a selective barrier that tightly regulates the movement of ions, molecules and cells between the blood and the CNS. Properties of drug such as molecular weight and affinity for a lipid environment affect their ability to pass BBB. The BBB allows small molecules to pass through but not macromolecules [12][13]. In addition, transport of even small drug molecules across the BBB is affected by helper molecules that move drugs from the blood to the brain. The drug may bind to targeted binding sites and to other tissue components that should be non-binding sites, affecting the final concentration–time profile of the drug at target site, which determines the pharmacodynamic effect over time [14]. Inside the brain, several factors can influence drug distribution, e.g., bulk flow of extracellular fluid (ECF), cerebrospinal fluid (CSF) and extra-cellular exchange. Furthermore, once having crossed the BBB, drug distribution within the ECF is also affected by the tortuosity of the tissue, leading to a relatively smaller effective diffusion [15][16].
Specially, the CNS tissue as an anisotropic composite material is a complex system for drug flow and distribution. Biomechanically, it can be broadly characterised by the stiff directional axons wrapped in insulating lipid-rich layers (myelin) that are supported by a soft matrix composed of glial cells and a network of biopolymers, the extracellular matrix (ECM). The directional axons can be a mechanical obstacle to drug diffusion and spatial distribution in the CNS tissue [17][18]. The presence of elongated axons can compel the drug particles to diffuse around fibre-like obstructions, hence the increased tortuosity and reduced effective diffusion. Furthermore, inside the tissue, it is the ECM that regulates the local transport of molecules. It provides selective filtering for nanoparticles (NPs) through its interactions. The filtering properties of the ECM can be deleterious for diffusion-based drug delivery and could perturb their flow behaviour and spatial distribution [19][20][21]. For example, it has been shown that presence of surface charge on particles can significantly suppress their diffusion in the ECM [22].
In brief, drug transport by diffusion often suffers from the loss of macromolecules across the BBB, binding to the receptors and uptake into cells. This issue complicates brain disease treatment by diffusion-based drug delivery therapies; therefore, no promising results have been achieved.
3. Infusion-Based Drug Delivery in CNS Tissue
Infusion-based transport provides an opportunity to overcome most of the challenges faced by drug delivery via diffusion in the CNS tissue. In contrast to the diffusion-based approach, which relies on concentration gradients, a positive pressure gradient drives the flow to the targeted area, also known as convective transport. There is a growing interest in understanding the pressure-driven drug delivery and underlying mechanisms for applications in CED-like technologies; however, challenges still exist. The main challenges include the unexpected relationship between drug distribution patterns and infusion parameters (such as the infusion rate, infusion volume and catheter angle), as well as backflow development, tissue edema and disruption of active tissue/BBB [7]. These, together with additional complexities due to brain tissue characteristics such as anisotropy and heterogeneity, lead to unexpected drug distribution patterns [23]. Progress mostly suffers from the lack of precise information and experimental data on how drug flow in CNS tissue is affected by its components (Figure 1), which could provide a fundamental mechanism to develop predictive models for drug flow and distribution. This problem is two-fold: (1) biomechanical aspects of infusion-based transport, i.e., understanding of flow field in CNS tissue and its corresponding mechanical response and (2) the molecular process involved between the drug and tissue components that influence drug flow and distribution. In this section, researchers will look into experimental studies focused on these aspects of infusion and discuss how a detailed knowledge of these processes, once developed, would eventually lead to predicting the drug flow and distribution in CNS tissue.
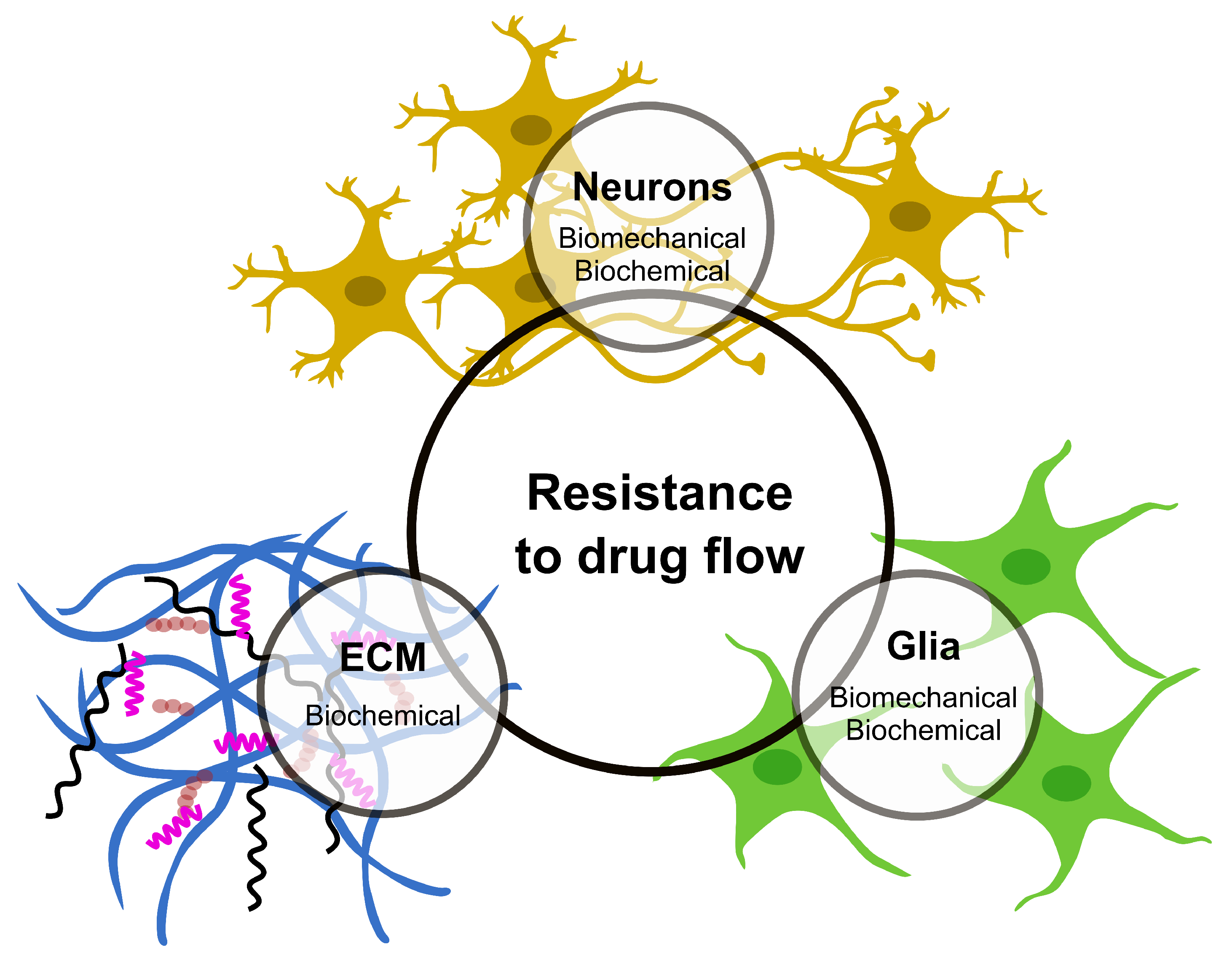
Figure 1. The CNS components that offer major resistance to drug flow and distribution.
This entry is adapted from the peer-reviewed paper 10.3390/ijms23063139
References
- Alphandéry, E. Glioblastoma treatments: An account of recent industrial developments. Front. Pharmacol. 2018, 9, 879.
- Bush, N.A.O.; Chang, S.M.; Berger, M.S. Current and future strategies for treatment of glioma. Neurosurg. Rev. 2017, 40, 1–14.
- Olesen, J.; Gustavsson, A.; Svensson, M.; Wittchen, H.U.; Jönsson, B. The economic cost of brain disorders in Europe. Eur. J. Neurol. 2012, 19, 155–162.
- Harder, B.G.; Blomquist, M.R.; Wang, J.; Kim, A.J.; Woodworth, G.F.; Winkles, J.A.; Loftus, J.C.; Tran, N.L. Developments in Blood-Brain Barrier Penetrance and Drug Repurposing for Improved Treatment of Glioblastoma. Front. Oncol. 2018, 8, 462.
- Weidle, U.H.; Niewohner, J.; Tiefenthaler, G. The blood-brain barrier challenge for the treatment of brain cancer, secondary brain metastases, and neurological diseases. Cancer Genom. Proteom. 2015, 12, 167–178.
- Yuan, F. Transvascular drug delivery in solid tumors. Semin. Radiat. Oncol. 1998, 8, 164–175.
- Mehta, A.M.; Sonabend, A.M.; Bruce, J.N. Convection-Enhanced Delivery. Neurotherapeutics 2017, 14, 358–371.
- Lonser, R.R.; Sarntinoranont, M.; Morrison, P.F.; Oldfield, E.H. Convection-enhanced delivery to the central nervous system. J. Neurosurg. 2015, 122, 697–706.
- Audette, M.A.; Bordas, S.P.; Blatt, J.E. Robotically Steered Needles: A Survey of Neurosurgical Applications and Technical Innovations. Robot. Surg. Res. Rev. 2020, 7, 1–23.
- Terzano, M.; Dini, D.; Rodriguez y Baena, F.; Spagnoli, A.; Oldfield, M. An adaptive finite element model for steerable needles. Biomech. Model. Mechanobiol. 2020, 19, 1809–1825.
- Azevedo, F.A.; Carvalho, L.R.; Grinberg, L.T.; Farfel, J.M.; Ferretti, R.E.; Leite, R.E.; Filho, W.J.; Lent, R.; Herculano-Houzel, S. Equal numbers of neuronal and nonneuronal cells make the human brain an isometrically scaled-up primate brain. The J. Comp. Neurol. 2009, 513, 532–541.
- Daneman, R. The blood-brain barrier in health and disease. Ann. Neurol. 2012, 72, 648–672.
- Daneman, R.; Prat, A. The Blood–Brain Barrier. Cold Spring Harb. Perspect. Biol. 2015, 7, a020412.
- Hammarlund-Udenaes, M.; Paalzow, L.K.; de Lange, E.C. Drug equilibration across the blood-brain barrier–pharmacokinetic considerations based on the microdialysis method. Pharm. Res. 1997, 14, 128–134.
- Nicholson, C. Diffusion and related transport mechanisms in brain tissue. Rep. Prog. Phys. 2001, 64, 815–884.
- Nicholson, C.; Phillips, J.M. Ion diffusion modified by tortuosity and volume fraction in the extracellular microenvironment of the rat cerebellum. J. Physiol. 1981, 321, 225–257.
- Jamal, A.; Mongelli, M.T.; Vidotto, M.; Madekurozwa, M.; Bernardini, A.; Overby, D.R.; De Momi, E.; Rodriguez y Baena, F.; Sherwood, J.M.; Dini, D. Infusion Mechanisms in Brain White Matter and Their Dependence on Microstructure: An Experimental Study of Hydraulic Permeability. IEEE Trans. Biomed. Eng. 2021, 68, 1229–1237.
- Vidotto, M.; Bernardini, A.; Trovatelli, M.; Momi, E.D.; Dini, D. On the Microstructural Origin of Brain White Matter Hydraulic Permeability. Proc. Natl. Acad. Sci. USA 2021, 118, e2105328118.
- Jain, R.K.; Stylianopoulos, T. Delivering nanomedicine to solid tumors. Nat. Rev. Clin. Oncol. 2010, 7, 653–664.
- Zhou, Y.; Chen, X.; Cao, J.; Gao, H. Overcoming the biological barriers in the tumor microenvironment for improving drug delivery and efficacy. J. Mater. Chem. B 2020, 8, 6765–6781.
- Bertrand, N.; Wu, J.; Xu, X.; Kamaly, N.; Farokhzad, O.C. Cancer nanotechnology: The impact of passive and active targeting in the era of modern cancer biology. Adv. Drug Deliv. Rev. 2014, 66, 2–25.
- Lieleg, O.; Baumgärtel, R.M.; Bausch, A.R. Selective Filtering of Particles by the Extracellular Matrix: An Electrostatic Bandpass. Biophys. J. 2009, 97, 1569–1577.
- Sarntinoranont, M.; Banerjee, R.K.; Lonser, R.R.; Morrison, P.F. A Computational Model of Direct Interstitial Infusion of Macromolecules into the Spinal Cord. Ann. Biomed. Eng. 2003, 31, 448–461.
- Sarntinoranont, M.; Banerjee, R.K.; Lonser, R.R.; Morrison, P.F. A Computational Model of Direct Interstitial Infusion of Macromolecules into the Spinal Cord. Ann. Biomed. Eng. 2003, 31, 448–461.
This entry is offline, you can click here to edit this entry!
