Metastasis is known as the most life-threatening event in cancer patients. In principle, the immune system can prevent tumor development. However, dysfunctional T cells may fail to eliminate the tumor cells effectively and provide additional survival advantages for tumor proliferation and metastasis. Constitutive activation of Ras-associated protein1 (Rap1) has not only led to T cell anergy, but also inhibited autophagy and supported cancer progression through various oncogenic events. Inhibition of Rap1 activity with its negative regulator, Rap1GAP, impairs tumor progression. However, active Rap1 reduces tumor invasion in some cancers, indicating that the pleiotropic effects of Rap1 signaling in cancers could be cancer-specific. All in all, targeting Rap1 signaling and its regulators could potentially control carcinogenesis, metastasis, chemoresistance and immune evasion. Rap1GAP could be a promising therapeutic target in combating cancer.
- Rap1
- Rap1GAP
- tumor initiation
- tumor progression
- cancer
Introduction
During each step of the metastatic cascade, the highly immunogenic tumor cells should be recognized and eliminated by the host immune system [1][2][3]. In general, the antigen-presenting cells (APCs) such as dendritic cells (DCs) phagocytose tumor antigens, and the fragmented antigens are generally known to be presented by major histocompatibility complex (MHC) class I and II to cytotoxic T lymphocytes (CTLs) and cluster of differentiation (CD)4+ T cells, respectively. Subsequently, the activated T cells should infiltrate into tumors in order to kill the cancer cells [4][5].
Cellular adhesion molecules such as integrins and the immunoglobulin superfamily may initiate T cell adhesion to APCs[6]. Particularly, integrin may mediate the infiltration of immune cells to the tumor site, the activation and proliferation of effector cells, and the formation of immunological synapse between immune cells and tumor cells[6]. The leukocyte function-associated antigen-1 (LFA-1) is a member of the β2 integrin family of adhesion receptor. It is also the predominant integrin in lymphocytes. It is an important component in T cell trafficking and extravasation[7] by binding to its ligand, the intercellular adhesion molecules-1 (ICAM-1)[6]. The LFA-1/ICAM-1 activates the formation of the immunological synapse via cell–cell interactions between immune effector cells and tumor cells (Figure 1).
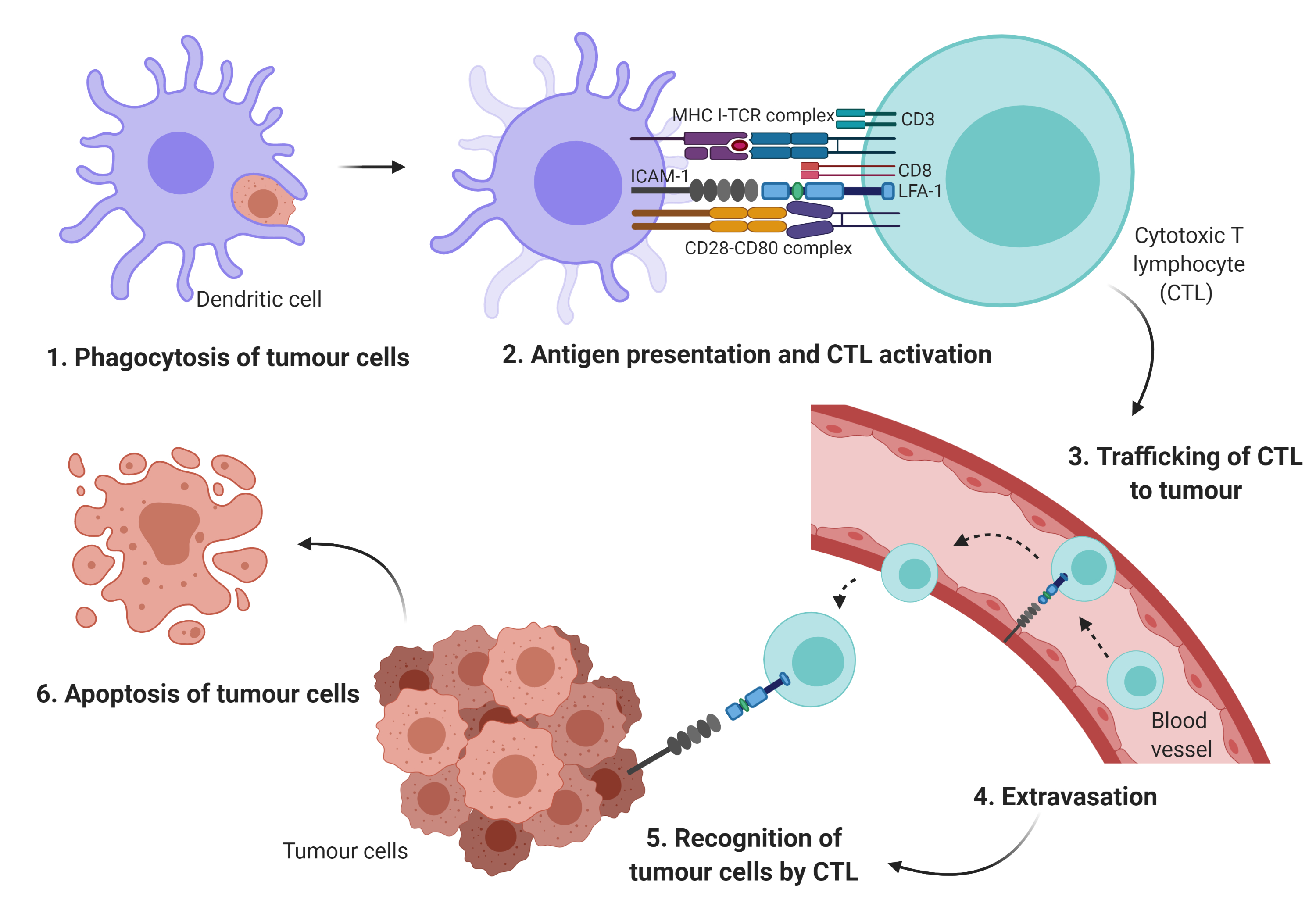
The small GTPase Ras-associated protein 1 (Rap1) is one of the main components of inside-out signaling cascades that plays a critical role in activating integrin[6][7]. Indeed, Rap1 signaling has been shown to play a role as a potent immunological regulator in modulating LFA-1-mediated adhesion in Jurkat T cells[9]. Activation of Rap1 in the T cells rapidly increased LFA-1 affinity, while overexpression of dominant-negative form of Rap1 (Rap1N17) or signal-induced proliferation-associated gene-1 (SPA-1) completely inhibited LFA-1 activation[10] and reduced adhesiveness and interleukin (IL)-2 production in Jurkat T cells[9]. Conversely, overexpression of Rap1 enhanced T cell activation and augmented IL-2 production[9]. In addition, in vivo study showed that increased active Rap1 in T lymphocytes was associated with enhanced T cell receptor (TCR)-mediated response and increased integrin activation[11]. Hence, these findings clearly indicate that Rap1 plays a crucial role in mediating integrin activation in T cells via inside-out signaling cascades
Biology of Rap1
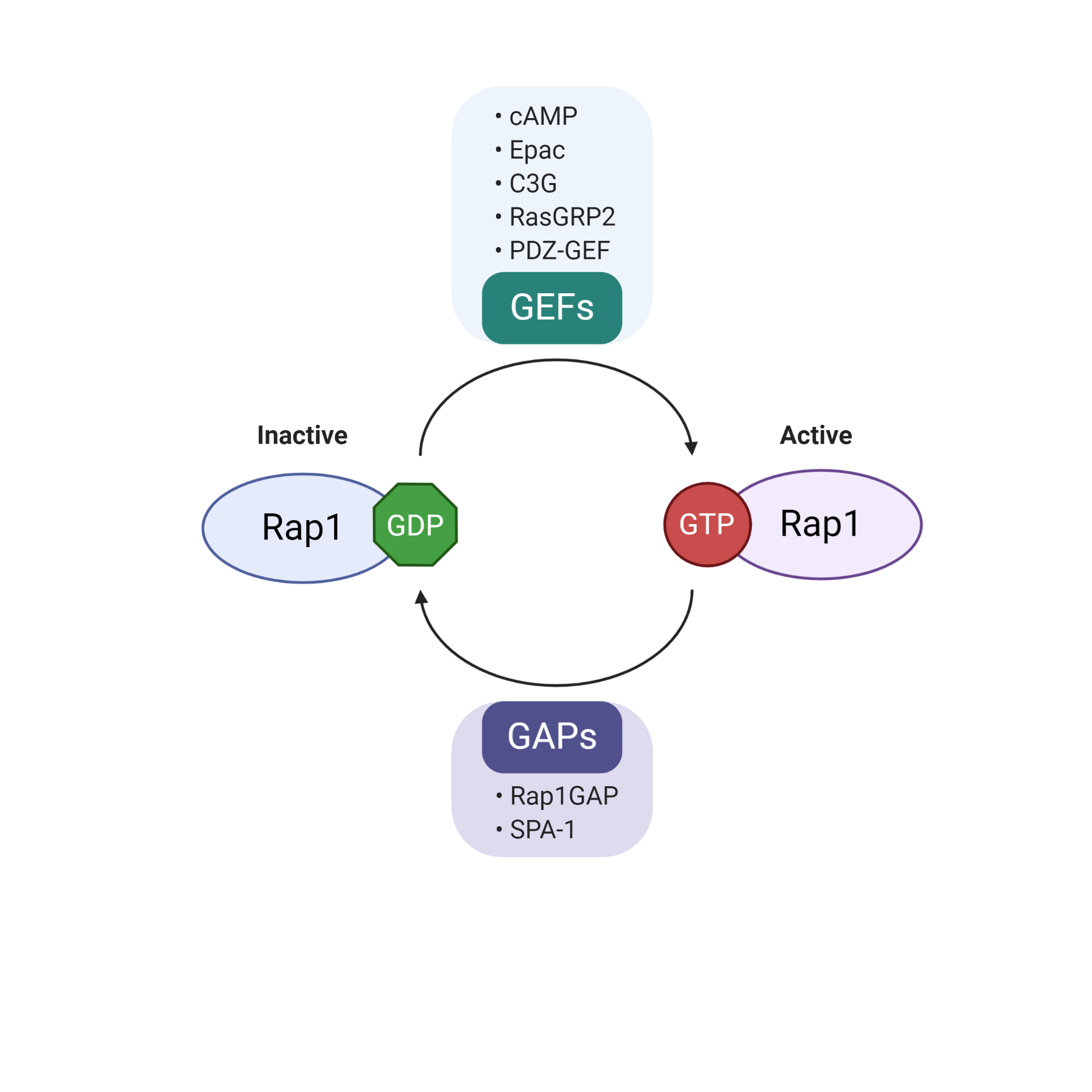
Rap1 is a member of the Ras-like small GTP binding protein family. Rap1 can bind to either guanosine triphosphate (GTP) or guanosine diphosphate (GDP). Rap1 activity is positively regulated by guanine nucleotide exchange factors (GEFs) and negatively regulated by GTPase-activating proteins (GAPs)[12]. GEFs promote dissociation of GDP, allowing loading of GTP to Rap1, resulting in the active form of GTP-bound Rap1 (Rap1-GTP). Conversely, Rap1-GTP is effectively hydrolyzed into inactive GDP-bound Rap1 (Rap1-GDP) in the presence of GAPs[12]. The well-characterized GEF family includes C3G (RapGEF1), RasGRP2, PDZ-GEF, cAMP and Epac[13]. In contrast to the diverse types of GEFs, there are only two groups of GAPs, Rap1GAP and SPA-1[14]. The regulation of the biological activity of Rap1 is summarized in Figure 2.
Rap1 Activation and Cancer
Rap1 signalling plays a diverse role in tumor initiation and progression (Figure 3). For instance, Rap1 activation induces tumor initiation and epithelial-mesenchymal transition (EMT) via Notch signaling[8]. EGFR and Src/FAK may be stimulated by the activated Rap1, leading to integrin-mediated cell adhesion in cancer. The adhesion of integrins to the extracellular matrix (ECM) is an essential step for tumor cell invasion and metastasis[8]. Src may activate MAPK/ERK to increase VEGF expression levels in the tumor cells, ultimately leading to angiogenesis. Src may also induce p130Cas and PD-L1 expression in the tumor cells[8]. The upregulation of MMP during Rap1 activation may trigger EMT in cancer and thus enhancing invasiveness and metastasis[8]. Therefore, Rap1 has rendered itself to be an attractive therapeutic target in cancer. However, it remains uncertain whether Rap1 signaling will positively or negatively regulate tumor progression as it showed to demonstrate contrasting effects on a tumor (Table 1). Therefore, further research is required to delineate its roles in cancers.
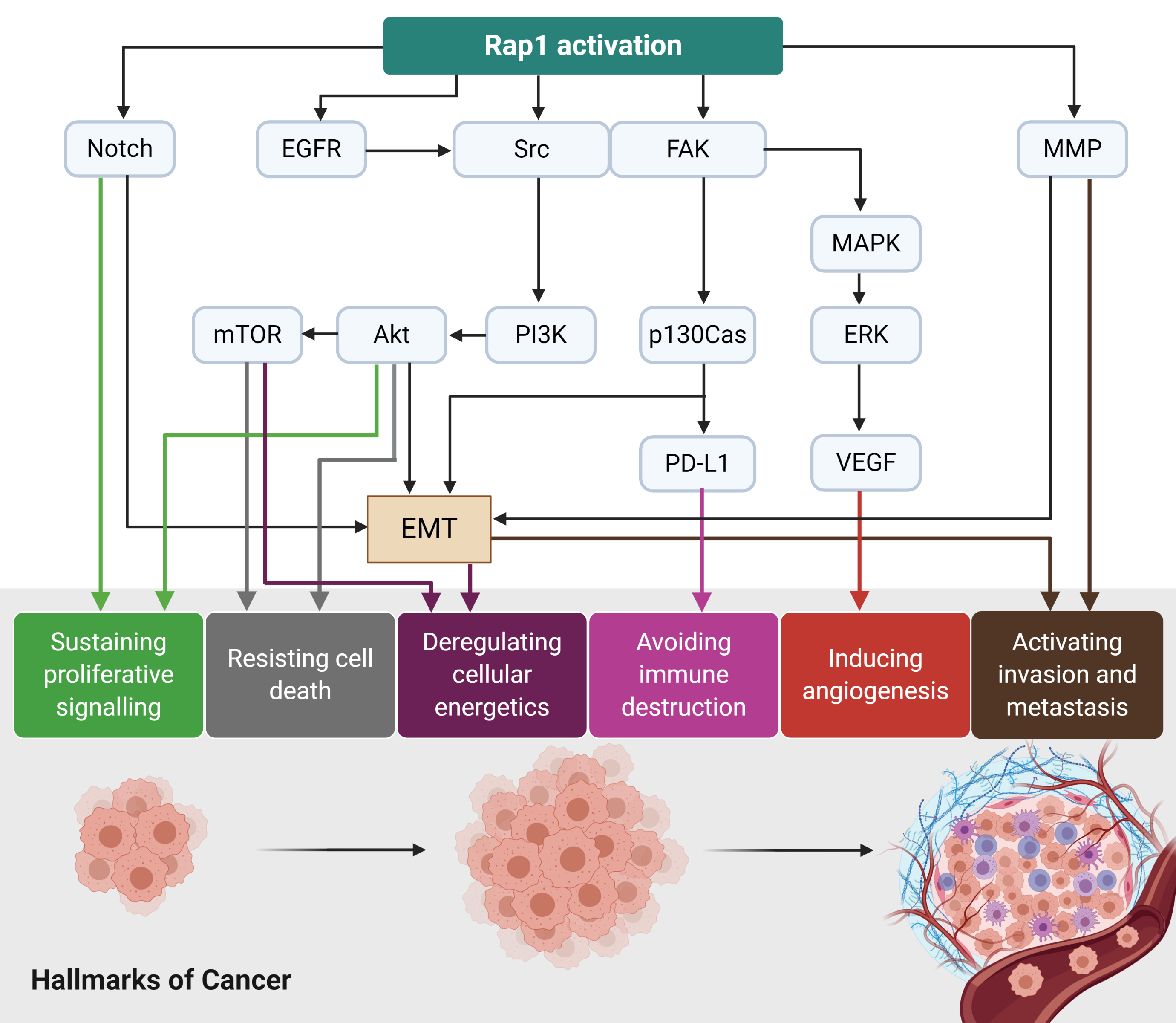
Table 1. Roles of Rap1 in tumor progression[8].
| Roles of Rap1 | Tumor Types | Signaling Molecules | Results | Reference |
|---|---|---|---|---|
| Tumor promoter | Glioblastoma | Rap1A, thrombin, β1-integrin | Knockdown of Rap1A reduced more than 70% of tumor growth compared to control | [15] |
| NSCLC | Rap1-GTP | Rap1-GTP depletion reduced growth of NSCLC cells and increased cisplatin sensitivity | [16] | |
| Melanoma | Rap1-GTP, p38 | Rap1-GTP expression promoted melanoma migration and invasion | [17] | |
| Breast cancer | Rap1-GTP, β1-integrin | Pharmacological inhibition of Rap1-GTP and β1-integrin reduced cell migration in breast cancer | [18] | |
| Rap1 | Expression of dominant-active Rap1 in breast epithelial cells increased invasiveness and tumorigenicity | [19] | ||
| Prostate cancer | Rap1A, α4, β3 -integrins | Rap1A activation increased expression of α4- and β3-integrins, leading to increased tumor cell invasion | [20] | |
| Pancreatic cancer | Rap1-GTP, EGFR | Rap1-GTP activation promoted migration and EGFR-mediated metastasis of pancreatic cancer cells | [21] | |
| Tumor suppressor | Bladder cancer | Rap1-GTP | Rap1-GTP activation suppressed migration of NBT-II bladder carcinoma cells | [22] |
Rap1 and Integrin
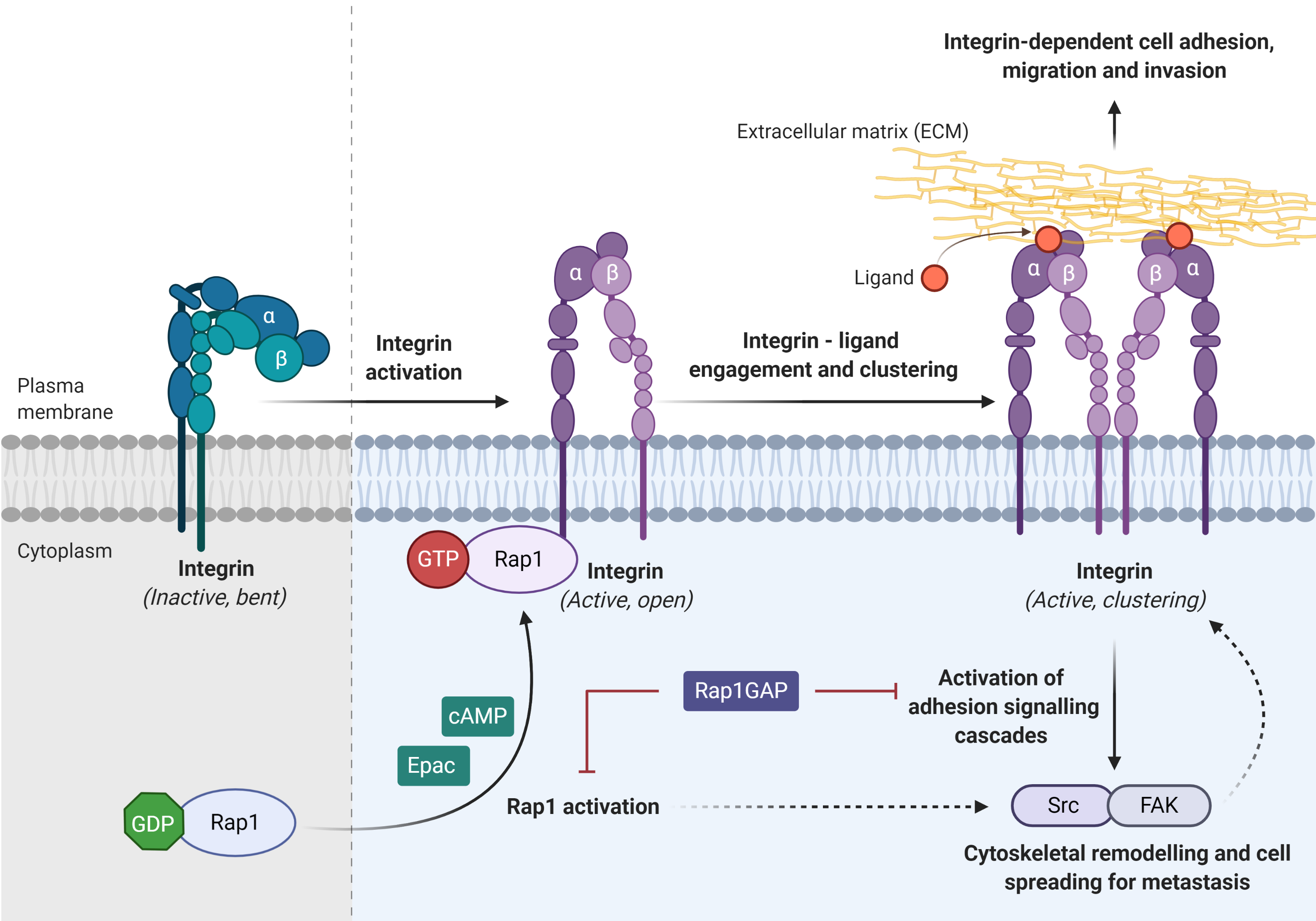
Integrin, the main cell adhesion receptor for the extracellular matrix (ECM), exists in different conformational states, and thus leads to the difference in its receptor affinity. An inactivated integrin (bent and closed) is associated with a low affinity for ECM ligand, whereas the active form (open) integrin is capable of binding to the ligand and clustering on the plasma membrane (adhesion)[8]. Rap1 mediates integrin-dependent biological processes in cancers, by which integrin adhesion activates signaling pathway such as Src/FAK to promote cytoskeletal remodeling, tumor migration, invasion, and metastasis (Figure 4)[8]. Integrin activation can be inhibited by Rap1GAP directly or indirectly[8].
Rap1 and Cadherin
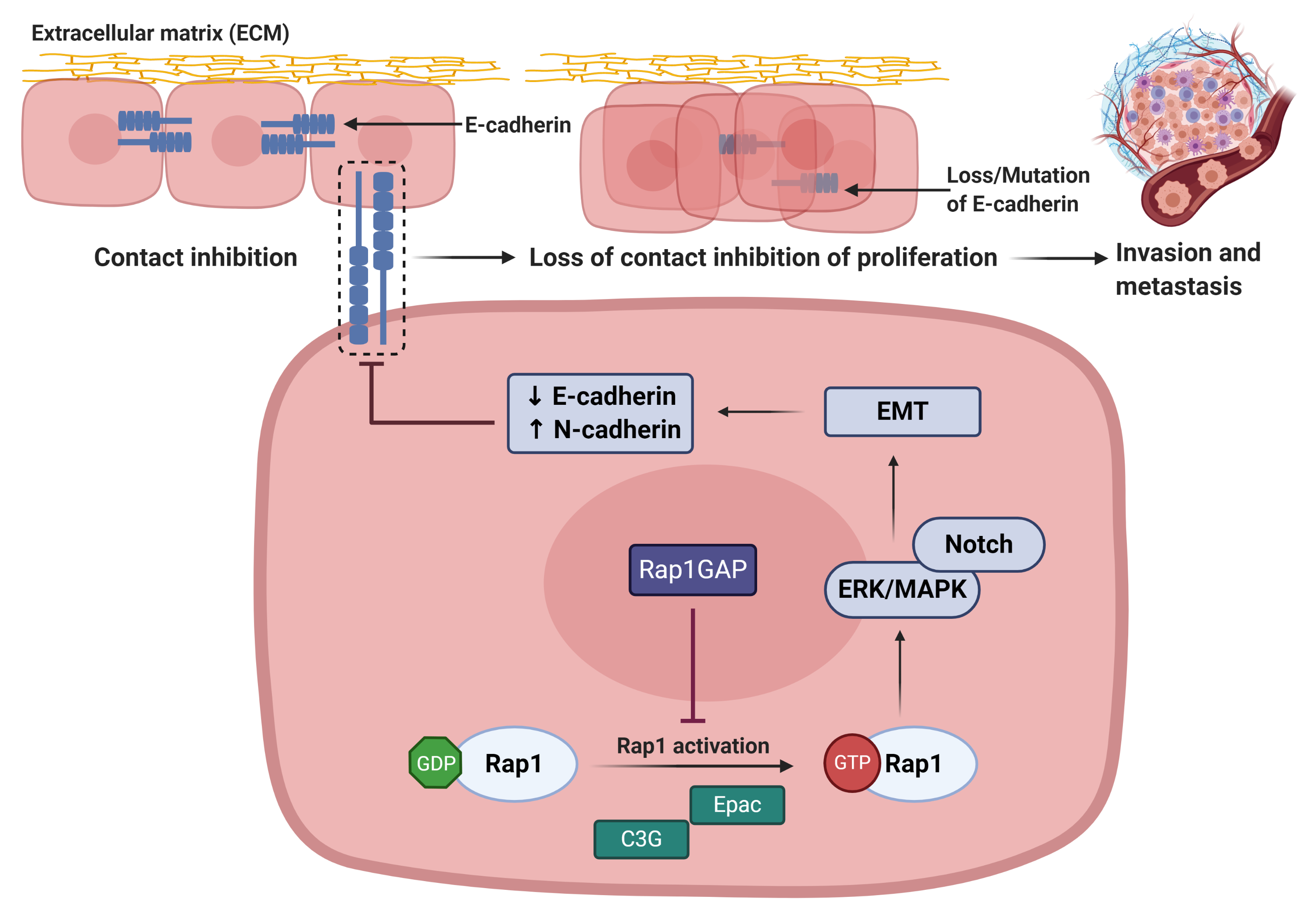
Rap 1 signalling plays important role in regulating cadherin expression (Figure 5). Rap1 activation leads to the reduction in E-cadherin expression, resulting in the loss of epithelial cell–cell adhesive junctions and contact inhibition of proliferation[8]. During the restoration of Rap1GAP expression in tumor cells, Rap1 will be inactivated and thus inhibiting tumor progression[8].
Figure 5: Rap1 promotes tumor progression via disruption of E-cadherin-mediated cell adhesion[8]
Rap1 Signaling Promotes Angiogenesis, Proliferation, and Survival
Rap1 signaling has been implicated in the regulation of angiogenesis, cell proliferation and migration in different cancers. In order to induce angiogenesis, VEGF increased Rap1 activity in an integrin-dependent manner. The use of β1 integrin antibody was shown to inhibit constitutive active Rap1-induced angiogenesis in endothelial cells[23]. Retroviral silencing of Rap1GAP greatly enhanced ERK/MAPK phosphorylation and activation, induced extensive cytoskeletal reorganization and expression of EMT features, and enhanced invasion in breast ductal carcinoma in situ (DCIS) cells[24]. Enforced re-expression of Rap1GAP in DCIS cells resulted in decreased Rap1 activation and a reversed mesenchymal to epithelial phenotype[24].
Therapeutic Potential of Rap1 Signaling in Cancers
Clinical studies have demonstrated that the loss of Rap1GAP expression was correlated with negative clinicopathological outcomes, such as invasion depth, lymph node metastasis, advanced TNM stages and poor overall survival (OS) as determined by Kaplan–Meier survival analyses[25][26]. Rescued expression of Rap1GAP in renal cell carcinoma cell lines using a demethylating drug, decitabine (5-azadC), resulted in a significant reduction in tumor invasiveness[27]. In pancreatic cancer, the use of a novel Epac-specific inhibitor (ESI-09), specifically inhibited Epac1-mediated Rap1 activation, ultimately resulting in impaired migration and invasion capability of cancer cells[28]. Taking all these into account, Rap1GAP holds the high potential to serve as a novel target for therapeutic intervention.
This entry is adapted from the peer-reviewed paper 10.3390/biomedicines8090334
References
- Li-Lian Gan; Ling-Wei Hii; Shew-Fung Wong; Chee-Onn Leong; Chun-Wai Mai; Molecular Mechanisms and Potential Therapeutic Reversal of Pancreatic Cancer-Induced Immune Evasion. Cancers 2020, 12, 1872, 10.3390/cancers12071872.
- Chin-King Looi; Felicia Fei-Lei Chung; Chee-Onn Leong; Shew-Fung Wong; Rozita Rosli; Chun-Wai Mai; Therapeutic challenges and current immunomodulatory strategies in targeting the immunosuppressive pancreatic tumor microenvironment. Journal of Experimental & Clinical Cancer Research 2019, 38, 162, 10.1186/s13046-019-1153-8.
- Felicia Fei-Lei Chung; Chee-Onn Leong; Chun-Wai Mai; Felicia Fei-Lei Chung And Chee-Onn Leong Chun-Wai Mai; Targeting Legumain As a Novel Therapeutic Strategy in Cancers. Current Drug Targets 2017, 18, 1259-1268, 10.2174/1389450117666161216125344.
- Daniel S Chen; Ira Mellman; Oncology Meets Immunology: The Cancer-Immunity Cycle. Immunity 2013, 39, 1-10, 10.1016/j.immuni.2013.07.012.
- Emma L Spurrell; Michelle Lockley; Adaptive immunity in cancer immunology and therapeutics. ecancermedicalscience 2014, 8, 441, 10.3332/ecancer.2014.441.
- Heidi Harjunpää; Marc Llort Asens; Carla Guenther; Susanna Carola Fagerholm; Cell Adhesion Molecules and Their Roles and Regulation in the Immune and Tumor Microenvironment. Frontiers in Immunology 2019, 10, 1078, 10.3389/fimmu.2019.01078.
- Navin Kumar Verma; Dermot Kelleher; Not Just an Adhesion Molecule: LFA-1 Contact Tunes the T Lymphocyte Program. The Journal of Immunology 2017, 199, 1213-1221, 10.4049/jimmunol.1700495.
- Chin-King Looi; Ling-Wei Hii; Siew Ching Ngai; Chee-Onn Leong; Chun-Wai Mai; The Role of Ras-Associated Protein 1 (Rap1) in Cancer: Bad Actor or Good Player?. Biomedicines 2020, 8, 334, 10.3390/biomedicines8090334.
- Koko Katagiri; Masakazu Hattori; Nagahiro Minato; Tatsuo Kinashi; Rap1 Functions as a Key Regulator of T-Cell and Antigen-Presenting Cell Interactions and Modulates T-Cell Responses. Molecular and Cellular Biology 2002, 22, 1001-1015, 10.1128/mcb.22.4.1001-1015.2002.
- Koko Katagiri; Masakazu Hattori; Nagahiro Minato; Shin-Kichi Irie; Kiyoshi Takatsu; Tatsuo Kinashi; Rap1 Is a Potent Activation Signal for Leukocyte Function-Associated Antigen 1 Distinct from Protein Kinase C and Phosphatidylinositol-3-OH Kinase. Molecular and Cellular Biology 2000, 20, 1956-1969, 10.1128/mcb.20.6.1956-1969.2000.
- Eric Sebzda; Madelon Bracke; Tamara Tugal; Nancy Hogg; Doreen A Cantrell; Rap1A positively regulates T cells via integrin activation rather than inhibiting lymphocyte signaling. Nature Immunology 2002, 3, 251-258, 10.1038/ni765.
- Zhang Yi-Lei; Wang Ruo-Chen; Cheng Ken; Ring Brian Z.; Su Li; Yilei Zhang; Ruo-Chen Wang; Ken Cheng; Brian Z. Ring; Li Su; et al. Roles of Rap1 signaling in tumor cell migration and invasion. Cancer Biology & Medicine 2017, 14, 90-99, 10.20892/j.issn.2095-3941.2016.0086.
- Selvi C. Jeyaraj; Nicholas T. Unger; Maqsood A. Chotani; Rap1 GTPases: An emerging role in the cardiovasculature. Life Sciences 2011, 88, 645-652, 10.1016/j.lfs.2011.01.023.
- Nagahiro Minato; Kohei Kometani; Masakazu Hattori; Regulation of Immune Responses and Hematopoiesis by the Rap1 Signal. Advances in Immunology 2007, 93, 229-264, 10.1016/s0065-2776(06)93006-5.
- Jacqueline Sayyah; Alena Bartakova; Nekeisha Nogal; Lawrence A. Quilliam; Dwayne G. Stupack; Joan Heller Brown; The Ras-related Protein, Rap1A, Mediates Thrombin-stimulated, Integrin-dependent Glioblastoma Cell Proliferation and Tumor Growth. Journal of Biological Chemistry 2014, 289, 17689-17698, 10.1074/jbc.m113.536227.
- Lu Xiao; Xiaoying Lan; Xianping Shi; Kai Zhao; Dongrui Wang; Xuejun Wang; Faqian Li; Hongbiao Huang; Jinbao Liu; Cytoplasmic RAP1 mediates cisplatin resistance of non-small cell lung cancer.. Cell Death & Disease 2017, 8, e2803-e2803, 10.1038/cddis.2017.210.
- Sitong Zhou; Yanhua Liang; Xi Zhang; Lexi Liao; Yao Yang; Wen Ouyang; Huaiyuan Xu; SHARPIN Promotes Melanoma Progression via Rap1 Signaling Pathway. Journal of Investigative Dermatology 2020, 140, 395-403.e6, 10.1016/j.jid.2019.07.696.
- Elaine A McSherry; K Brennan; Lance Hudson; A.D.K. Hill; Ann M Hopkins; Breast cancer cell migration is regulated through junctional adhesion molecule-A-mediated activation of Rap1 GTPase. Breast Cancer Research 2011, 13, R31-R31, 10.1186/bcr2853.
- Masahiko Itoh; Celeste M. Nelson; Connie A. Myers; Mina J. Bissell; Rap1 integrates tissue polarity, lumen formation, and tumorigenic potential in human breast epithelial cells.. Cancer Research 2007, 67, 4759-66, 10.1158/0008-5472.CAN-06-4246.
- Candice L. Bailey; Patrick Kelly; Patrick J. Casey; Activation of Rap1 Promotes Prostate Cancer Metastasis. Cancer Research 2009, 69, 4962-4968, 10.1158/0008-5472.can-08-4269.
- Miller Huang; Sudarshan Anand; Eric A. Murphy; Jay S. Desgrosellier; Dwayne G. Stupack; Sanford J. Shattil; David D. Schlaepfer; David A. Cheresh; EGFR-dependent pancreatic carcinoma cell metastasis through Rap1 activation.. Oncogene 2011, 31, 2783-93, 10.1038/onc.2011.450.
- Ana M. Vallés; Maud Beuvin; Brigitte Boyer; Activation of Rac1 by Paxillin-Crk-DOCK180 Signaling Complex Is Antagonized by Rap1 in Migrating NBT-II Cells. Journal of Biological Chemistry 2004, 279, 44490-44496, 10.1074/jbc.m405144200.
- Guillaume Carmona; Stephan Göttig; Alessia Orlandi; Jürgen Scheele; Tobias Bäuerle; Manfred Jugold; Fabian Kiessling; Reinhard Henschler; Andreas M. Zeiher; Stefanie Dimmeler; et al. Role of the small GTPase Rap1 for integrin activity regulation in endothelial cells and angiogenesis. Blood 2009, 113, 488-497, 10.1182/blood-2008-02-138438.
- Seema Shah; Ethan J. Brock; Ryan M. Jackson; Kyungmin Ji; Julie L. Boerner; Bonnie F. Sloane; Raymond R. Mattingly; Downregulation of Rap1Gap: A Switch from DCIS to Invasive Breast Carcinoma via ERK/MAPK Activation.. Neoplasia 2018, 20, 951-963, 10.1016/j.neo.2018.07.002.
- Ya Yang; Jia Zhang; Yan Yan; Hui Cai; Min Li; Kai Sun; Jizhao Wang; Xu Liu; Jiansheng Wang; Xiaoyi Duan; et al. Low expression of Rap1GAP is associated with epithelial-mesenchymal transition (EMT) and poor prognosis in gastric cancer. Oncotarget 2016, 8, 8057-8068, 10.18632/oncotarget.14074.
- Wei‑Li Gao; Guo‑Chao Ye; Li‑Wei Liu; Lu Wei; The downregulation of Rap1 GTPase-activating protein is associated with a poor prognosis in colorectal cancer and may impact on tumor progression. Oncology Letters 2018, 15, 7661-7668, 10.3892/ol.2018.8305.
- Wan-Ju Kim; Zachary Gersey; Yehia Daaka; Rap1GAP regulates renal cell carcinoma invasion.. Cancer Letters 2012, 320, 65-71, 10.1016/j.canlet.2012.01.022.
- Muayad Almahariq; Tamara Tsalkova; Fang C. Mei; Haijun Chen; Jia Zhou; Sarita K. Sastry; Frank Schwede; Xiaodong Cheng; A Novel EPAC-Specific Inhibitor Suppresses Pancreatic Cancer Cell Migration and Invasion. Molecular Pharmacology 2012, 83, 122-128, 10.1124/mol.112.080689.