Hypoxic-ischemic encephalopathy (HIE) following perinatal asphyxia is a major cause of neurological sequelae in term and near-term infants. Despite therapeutic hypothermia, a significant number of infants still have adverse outcomes. Neuroimaging is the standard of care in infants with HIE to determine the nature and timing of the injury, guide further treatment, and predict neurodevelopmental outcomes. Cranial ultrasonography is helpful to assess the brain before initiation of therapeutic hypothermia to look for abnormalities suggestive of antenatal onset of injury or HIE mimics. However, magnetic resonance imaging (MRI) which includes diffusion-weighted imaging has become the gold standard to assess brain injury in newborns with HIE, and has an excellent prognostic utility. Magnetic resonance spectroscopy provides complementary metabolic information and has also been shown to be a reliable prognostic biomarker. Advanced imaging modalities, such as diffusion tensor imaging and arterial spin labeling, are increasingly being used to gain further information about the etiology and prognosis of brain injury in infants with HIE due to perinatal asphyxia.
- perinatal asphyxia
- hypoxic-ischemic encephalopathy
- neonatal encephalopathy
- neonatal neuroimaging
- magnetic resonance imaging
- diffusion-weighted imaging
- magnetic resonance spectroscopy
- outcome prediction
1. Brain Injury Patterns in HIE
1.1. Basal Ganglia and Thalami (BGT) Predominant Pattern of Injury
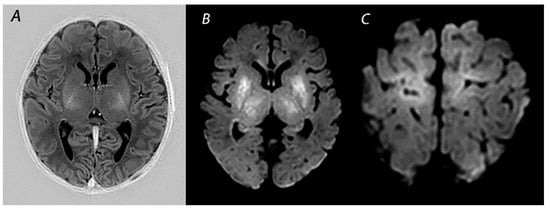
1.2. White Matter/Watershed (WM/WS) Predominant Pattern of Injury
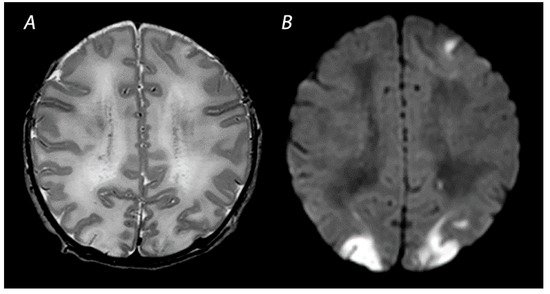
1.3. Near Total Injury
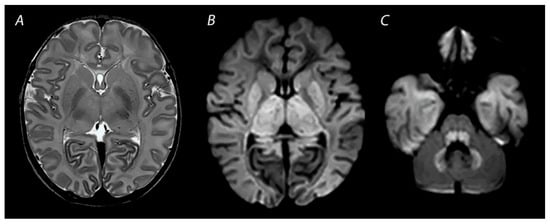
1.4. Other Injury Associated with HIE
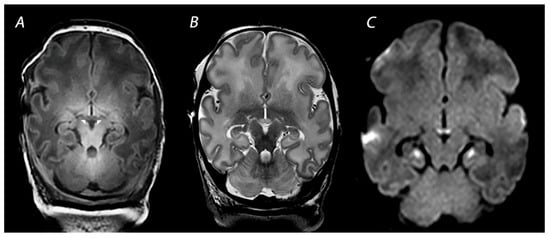
2. Magnetic Resonance Imaging in Infants with HIE
2.1. Conventional MRI
2.2. Diffusion Weighted Imaging
2.3. Susceptibility Weighted Imaging
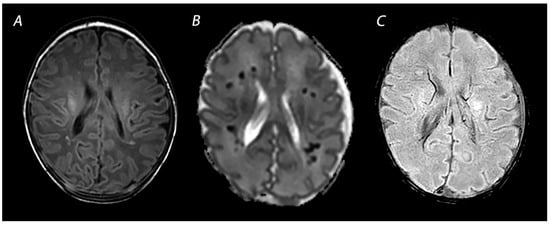
3. Advanced Imaging Modalities
3.1. Diffusion Tensor Imaging
3.2. Arterial Spin Labeling
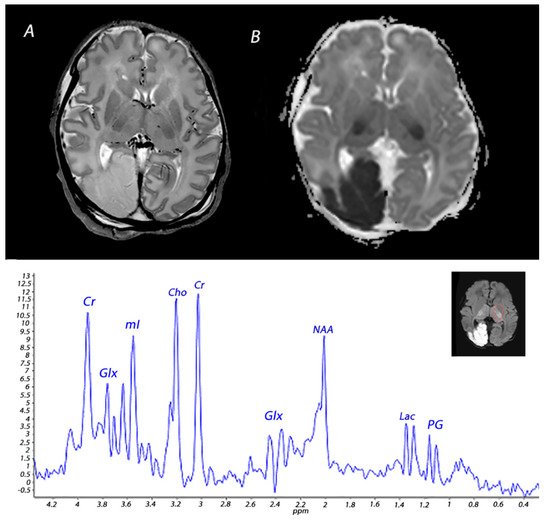
4. Magnetic Resonance Spectroscopy
This entry is adapted from the peer-reviewed paper 10.3390/diagnostics12030645
References
- Gunn, A.J.; Bennet, L. Fetal hypoxia insults and patterns of brain injury: Insights from animal models. Clin. Perinatol. 2009, 36, 579–593.
- Okereafor, A.; Allsop, J.; Counsell, S.J.; Fitzpatrick, J.; Azzopardi, D.; Rutherford, M.A.; Cowan, F.M. Patterns of brain injury in neonates exposed to perinatal sentinel events. Pediatrics 2008, 121, 906–914.
- Martinez-Biarge, M.; Diez-Sebastian, J.; Kapellou, O.; Gindner, D.; Allsop, J.M.; Rutherford, M.A.; Cowan, F.M. Predicting motor outcome and death in term hypoxic-ischemic encephalopathy. Neurology 2011, 76, 2055–2061.
- Martinez-Biarge, M.; Diez-Sebastian, J.; Rutherford, M.A.; Cowan, F.M. Outcomes after central grey matter injury in term perinatal hypoxic-ischaemic encephalopathy. Early Hum. Dev. 2010, 86, 675–682.
- Volpe, J.J.; Pasternak, J.F. Parasagittal cerebral injury in neonatal hypoxic-ischemic encephalopathy: Clinical and neuroradiologic features. J. Pediatr. 1977, 91, 472–476.
- Miller, S.; Ramaswamy, V.; Michelson, D.; Barkovich, A.J.; Holshouser, B.; Wycliffe, N.; Glidden, D.; Deming, D.; Partridge, J.C.; Wu, Y.W.; et al. Patterns of brain injury in term neonatal encephalopathy. J. Pediatr. 2005, 146, 453–460.
- Sato, Y.; Hayakawa, M.; Iwata, O.; Okumura, A.; Kato, T.; Hayakawa, F.; Kubota, T.; Maruyama, K.; Hasegawa, M.; Sato, M.; et al. Delayed neurological signs following isolated parasagittal injury in asphyxia at term. Eur. J. Paediatr. Neurol. 2008, 12, 359–365.
- Martinez-Biarge, M.; Bregant, T.; Wusthoff, C.; Chew, A.T.; Diez-Sebastian, J.; Rutherford, M.A.; Cowan, F.M. White matter and cortical injury in hypoxic-ischemic encephalopathy: Antecedent factors and 2-year outcome. J. Pediatr. 2012, 161, 799–807.
- Harteman, J.C.; Groenendaal, F.; Toet, M.C.; Benders, M.J.; Van Haastert, I.C.; Nievelstein, R.A.; Koopman-Esseboom, C.; De Vries, L.S. Diffusion-weighted imaging changes in cerebral watershed distribution following neonatal encephalopathy are not invariably associated with an adverse outcome. Dev. Med. Child Neurol. 2013, 55, 642–653.
- Perez, A.; Ritter, S.; Brotschi, B.; Werner, H.; Caflisch, J.; Martin, E.; Latal, B. Long-term neurodevelopmental outcome with hypoxic-ischemic encephalopathy. J. Pediatr. 2013, 163, 454–459.
- Lee, B.L.; Gano, D.; Rogers, E.E.; Xu, D.; Cox, S.; Barkovich, A.J.; Li, Y.; Ferriero, D.M.; Glass, H.C. Long-term cognitive outcomes in term newborns with watershed injury caused by neonatal encephalopathy. Pediatr. Res. 2021. Online ahead of print.
- Hayman, M.; van Wezel-Meijler, G.; van Straaten, H.; Brilstra, E.; Groenendaal, F.; de Vries, L.S. Punctate white-matter lesions in the full-term newborn: Underlying aetiology and outcome. Eur. J. Paediatr. Neurol. 2019, 23, 280–287.
- Li, A.M.; Chau, V.; Poskitt, K.J.; Sargent, M.A.; Lupton, B.A.; Hill, A.; Roland, E.; Miller, S.P. White matter injury in term newborns with neonatal encephalopathy. Pediatr. Res. 2009, 65, 85–89.
- Vermeulen, R.J.; Fetter, W.P.; Hendrikx, L.; Van Schie, P.E.; van der Knaap, M.S.; Barkhof, F. Diffusion-weighted MRI in severe neonatal hypoxic ischaemia: The white cerebrum. Neuropediatrics 2003, 34, 72–76.
- Annink, K.V.; Meerts, L.; van der Aa, N.E.; Alderliesten, T.; Nikkels, P.G.J.; Nijboer, C.H.A.; Groenendaal, F.; de Vries, L.S.; Benders, M.J.N.L.; Hoebeek, F.E.; et al. Cerebellar injury in term neonates with hypoxic-ischemic encephalopathy is underestimated. Pediatr. Res. 2021, 89, 1171–1178.
- Lemmon, M.E.; Wagner, M.W.; Bosemani, T.; Carson, K.A.; Northington, F.J.; Huisman, T.A.; Poretti, A. Diffusion tensor imaging detects occult cerebellar injury in severe neonatal hypoxic-ischemic encephalopathy. Dev. Neurosci. 2017, 39, 207–214.
- Radicioni, M.; Bini, V.; Chiarini, P.; Fantauzzi, A.; Leone, F.; Scattoni, R.; Camerini, P.G. Cerebral sinovenous thrombosis in the asphyxiated cooled infants: A prospective observational study. Pediatr. Neurol. 2017, 66, 63–68.
- Cowan, F.; Rutherford, M.; Groenendaal, F.; Eken, P.; Mercuri, E.; Bydder, G.M.; Meiners, L.C.; Dubowitz, L.M.; de Vries, L.S. Origin and timing of brain lesions in term infants with neonatal encephalopathy. Lancet 2003, 361, 736–742.
- Ramaswamy, V.; Miller, S.P.; Barkovich, A.J.; Partridge, J.C.; Ferriero, D.M. Perinatal stroke in term infants with neonatal encephalopathy. Neurology 2004, 62, 2088–2091.
- Adhami, F.; Liao, G.; Morozov, Y.M.; Schloemer, A.; Schmithorst, V.J.; Lorenz, J.N.; Dunn, R.S.; Vorhees, C.V.; Wills-Karp, M.; Degen, J.L.; et al. Cerebral ischemia-hypoxia induces intravascular coagulation and autophagy. Am. J. Pathol. 2006, 169, 566–583.
- Michoulas, A.; Basheer, S.N.; Roland, E.H.; Poskitt, K.; Miller, S.; Hill, A. The role of hypoxia-ischemia in term newborns with arterial stroke. Pediatr. Neurol. 2011, 44, 254–258.
- Harbert, M.J.; Tam, E.W.Y.; Glass, H.C.; Bonifacio, S.L.; Haeusslein, L.A.; Barkovich, A.J.; Jeremy, R.J.; Rogers, E.E.; Glidden, D.; Ferriero, D.M. Hypothermia is correlated with seizure absence in perinatal stroke. J. Child Neurol. 2011, 26, 1126–1130.
- Alderliesten, T.; Nikkels, P.G.; Benders, M.J.; de Vries, L.S.; Groenendaal, F. Antemortem cranial MRI compared with postmortem histopathologic examination of the brain in term infants with neonatal encephalopathy following perinatal asphyxia. Arch. Dis. Child. Fetal Neonatal Ed. 2012, 98, F304–F309.
- Annink, K.V.; De Vries, L.S.; Groenendaal, F.; Heuvel, M.P.V.D.; Van Haren, N.E.M.; Swaab, H.; Van Handel, M.; Jongmans, M.J.; Benders, M.J.; Van Der Aa, N.E. The long-term effect of perinatal asphyxia on hippocampal volumes. Pediatr. Res. 2019, 85, 43–49.
- Molavi, M.; Vann, S.D.; de Vries, L.S.; Groenendaal, F.; Lequin, M. Signal change in the mammillary bodies after perinatal asphyxia. AJNR Am. J. Neuroradiol. 2019, 40, 1829–1834.
- Lequin, M.; Steggerda, S.; Severino, M.; Tortora, D.; Parodi, A.; Ramenghi, L.A.; Groenendaal, F.; Meys, K.M.; Benders, M.J.; de Vries, L.S.; et al. Mammillary body injury in neonatal encephalopathy: A multicentre, retrospective study. Pediatr. Res. 2021. Online ahead of print.
- Annink, K.V.; de Vries, L.S.; Groenendaal, F.; Eijsermans, R.M.J.C.; Mocking, M.; van Schooneveld, M.M.J.; Dudink, J.; van Straaten, H.L.M.; Benders, M.J.N.L.; Lequin, M.; et al. Mammillary body atrophy and other MRI correlates of school-age outcome following neonatal hypoxic-ischemic encephalopathy. Sci. Rep. 2021, 11, 5017.
- Barkovich, A.J.; Truwit, C.L. Brain damage from perinatal asphyxia: Correlation of MR findings with gestational age. AJNR Am. J. Neuroradiol. 1990, 11, 1087–1096.
- Shroff, M.M.; Soares-Fernandes, J.P.; Whyte, H.; Raybaud, C. MR imaging for diagnostic evaluation of encephalopathy in the newborn. Radiographics 2010, 30, 763–780.
- Barkovich, A.J.; Westmark, K.; Partridge, C.; Sola, A.; Ferriero, D.M. Perinatal asphyxia—MR findings in the first 10 days. Am. J. Neuroradiol. 1995, 16, 427–438.
- Rutherford, M.A.; Pennock, J.M.; Counsell, S.J.; Mercuri, E.; Cowan, F.M.; Dubowitz, L.M.S.; Edwards, A.D. Abnormal magnetic resonance signal in the internal capsule predicts poor neurodevelopmental outcome in infants with hypoxic-ischemic encephalopathy. Pediatrics 1998, 102 Pt 1, 323–328.
- Sener, R.N. Diffusion MRI: Apparent diffusion coefficient (ADC) values in the normal brain and a classification of brain disorders based on ADC values. Comput. Med. Imaging Graph. 2001, 25, 299–326.
- Cowan, F.M.; Pennock, J.M.; Hanrahan, J.D.; Manji, K.P.; Edwards, A.D. Early detection of cerebral infarction and hypoxic ischemic encephalopathy in neonates using diffusion-weighted magnetic resonance imaging. Neuropediatrics 1994, 25, 172–175.
- Robertson, R.L.; Ben-Sira, L.; Barnes, P.D.; Mulkern, R.V.; Robson, C.D.; Maier, S.E.; Rivkin, M.J.; Du Plessis, A.J. MR line-scan diffusion-weighted imaging of term neonates with perinatal brain ischemia. AJNR Am. J. Neuroradiol. 1999, 20, 1658–1670.
- Bednarek, N.; Mathur, A.; Inder, T.; Wilkinson, J.; Neil, J.; Shimony, J. Impact of therapeutic hypothermia on MRI diffusion changes in neonatal encephalopathy. Neurology 2012, 78, 1420–1427.
- Haacke, E.M.; Mittal, S.; Wu, Z.; Neelavalli, J.; Cheng, Y.C. Susceptibility-weighted imaging: Technical aspects and clinical applications, part 1. AJNR Am. J. Neuroradiol. 2009, 30, 19–30.
- Bosemani, T.; Poretti, A.; Huisman, T.A. Susceptibility-weighted imaging in pediatric neuroimaging. J. Magn. Reson. Imaging 2014, 40, 530–544.
- Kitamura, G.; Kido, D.; Wycliffe, N.; Jacobson, J.P.; Oyoyo, U.; Ashwal, S. Hypoxic-ischemic injury: Utility of susceptibility-weighted imaging. Pediatr. Neurol. 2011, 45, 220–224.
- Messina, S.A.; Poretti, A.; Tekes, A.; Robertson, C.; Johnston, M.V.; Huisman, T.A. Early predictive value of susceptibility weighted imaging (SWI) in pediatric hypoxic-ischemic injury. J. Neuroimaging 2014, 24, 528–530.
- Smith, S.M.; Jenkinson, M.; Johansen-Berg, H.; Rueckert, D.; Nichols, T.E.; Mackay, C.; Watkins, K.; Ciccarelli, O.; Cader, M.Z.; Matthews, P.M.; et al. Tract-based spatial statistics: Voxelwise analysis of multi-subject diffusion data. Neuroimage 2006, 31, 1487–1505.
- Lally, P.J.; Montaldo, P.; Oliveira, V.; Soe, A.; Swamy, R.; Bassett, P.; Mendoza, J.; Atreja, G.; Kariholu, U.; Pattnayak, S.; et al. Magnetic resonance spectroscopy assessment of brain injury after moderate hypothermia in neonatal encephalopathy: A prospective multicentre cohort study. Lancet Neurol. 2019, 18, 35–45.
- Porter, E.J.; Counsell, S.J.; Edwards, A.D.; Allsop, J.; Azzopardi, D. Tract-based spatial statistics of magnetic resonance images to assess disease and treatment effects in perinatal asphyxial encephalopathy. Pediatr. Res. 2010, 68, 205–209.
- Tusor, N.; Wusthoff, C.; Smee, N.; Merchant, N.; Arichi, T.; Allsop, J.M.; Cowan, F.M.; Azzopardi, D.; Edwards, A.D.; Counsell, S.J. Prediction of neurodevelopmental outcome after hypoxic-ischemic encephalopathy treated with hypothermia by diffusion tensor imaging analyzed using tract-based spatial statistics. Pediatr. Res. 2012, 72, 63–69.
- Petersen, E.T.; Zimine, I.; Ho, Y.C.; Golay, X. Non-invasive measurement of perfusion: A critical review of arterial spin labelling techniques. Br. J. Radiol. 2006, 79, 688–701.
- Kleuskens, D.G.; Goncalves Costa, F.; Annink, K.V.; van den Hoogen, A.; Alderliesten, T.; Groenendaal, F.; Benders, M.J.; Dudink, J. Pathophysiology of cerebral hyperperfusion in term neonates with hypoxic-ischemic encephalopathy: A systematic review for future research. Front. Pediatr. 2021, 9, 631258.
- De Vis, J.B.; Hendrikse, J.; Petersen, E.T.; de Vries, L.S.; van Bel, F.; Alderliesten, T.; Negro, S.; Groenendaal, F.; Benders, M.J. Arterial spin-labelling perfusion MRI and outcome in neonates with hypoxic-ischemic encephalopathy. Eur. Radiol. 2015, 25, 113–121.
- Wintermark, P.; Hansen, A.; Gregas, M.C.; Soul, J.; Labrecque, M.; Robertson, R.L.; Warfield, S.K. Brain perfusion in asphyxiated newborns treated with therapeutic hypothermia. AJNR Am. J. Neuroradiol. 2011, 32, 2023–2029.
- Meng, L.; Wang, Q.; Li, Y.; Ma, X.; Li, W.; Wang, Q. Diagnostic performance of arterial spin-labeled perfusion imaging and diffusion-weighted imaging in full-term neonatal hypoxic-ischemic encephalopathy. J. Integr. Neurosci. 2021, 20, 985–991.
- Proisy, M.; Corouge, I.; Legouhy, A.; Nicolas, A.; Charon, V.; Mazille, N.; Leroux, S.; Bruneau, B.; Barillot, C.; Ferré, J.-C. Changes in brain perfusion in successive arterial spin labeling MRI scans in neonates with hypoxic-ischemic encephalopathy. Neuroimage Clin. 2019, 24, 101939.
- Peden, C.J.; Cowan, F.M.; Bryant, D.J.; Sargentoni, J.; Cox, I.J.; Menon, D.K.; Gadian, D.G.; Bell, J.D.; Dubowitz, L.M. Proton MR spectroscopy of the brain in infants. J. Comput. Assist. Tomogr. 1990, 14, 886–894.
- Alderliesten, T.; de Vries, L.S.; Benders, M.J.; Koopman, C.; Groenendaal, F. MR imaging and outcome of term neonates with perinatal asphyxia: Value of diffusion-weighted MR imaging and (1)H MR spectroscopy. Radiology 2011, 261, 235–242.
- Thayyil, S.; Chandrasekaran, M.; Taylor, A.; Bainbridge, A.; Cady, E.B.; Chong, W.K.K.; Murad, S.; Omar, R.Z.; Robertson, N.J. Cerebral magnetic resonance biomarkers in neonatal encephalopathy: A meta-analysis. Pediatrics 2010, 125, e382–e395.
- Schmitz, B.; Wang, X.; Barker, P.B.; Pilatus, U.; Bronzlik, P.; Dadak, M.; Kahl, K.G.; Lanfermann, H.; Ding, X.-Q. Effects of aging on the human brain: A proton and phosphorus MR spectroscopy study at 3T. J. Neuroimaging 2018, 28, 416–421.
- Shibasaki, J.; Aida, N.; Morisaki, N.; Tomiyasu, M.; Nishi, Y.; Toyoshima, K. Changes in brain metabolite concentrations after neonatal hypoxic-ischemic encephalopathy. Radiology 2018, 288, 840–848.
- Toft, P.B.; Leth, H.; Lou, H.C.; Pryds, O.; Henriksen, O. Metabolite concentrations in the developing brain estimated with proton MR spectroscopy. J. Magn. Reson. Imaging 1994, 4, 674–680.
- Roelants-Van Rijn, A.M.; Van der Grond, J.; De Vries, L.S.; Groenendaal, F. Value of H-1-MRS using different echo times in neonates with cerebral hypoxia-ischemia. Pediatr. Res. 2001, 49, 356–362.
- Wu, T.W.; Tamrazi, B.; Hsu, K.H.; Ho, E.; Reitman, A.J.; Borzage, M.; Blüml, S.; Wisnowski, J.L. Cerebral lactate concentration in neonatal hypoxic-ischemic encephalopathy: In relation to time, characteristic of injury, and serum lactate concentration. Front. Neurol. 2018, 9, 293.
- Shibasaki, J.; Niwa, T.; Piedvache, A.; Tomiyasu, M.; Morisaki, N.; Fujii, Y.; Toyoshima, K.; Aida, N. Comparison of predictive values of magnetic resonance biomarkers based on scan timing in neonatal encephalopathy following therapeutic hypothermia. J. Pediatr. 2021, 239, 101–109.e4.
- Leth, H.; Toft, P.B.; Pryds, O.; Peitersen, B.; Lou, H.C.; Henriksen, O. Brain lactate in preterm and growth-retarded neonates. Acta Paediatr. 1995, 84, 495–499.
- Roelants-van Rijn, A.M.; van der Grond, J.; Stigter, R.H.; de Vries, L.S.; Groenendaal, F. Cerebral structure and metabolism and long-term outcome in small-for-gestational-age preterm neonates. Pediatr. Res. 2004, 56, 285–290.
- Moorcraft, J.; Bolas, N.M.; Ives, N.K.; Ouwerkerk, R.; Smyth, J.; Rajagopalan, B.; Hope, P.L.; Radda, G.K. Global and depth resolved phosphorus magnetic resonance spectroscopy to predict outcome after birth asphyxia. Arch. Dis. Child. 1991, 66, 1119–1123.
- Roth, S.C.; Edwards, A.D.; Cady, E.B.; Delpy, D.T.; Wyatt, J.S.; Azzopardi, D.; Baudin, J.; Townsend, J.; Stewart, A.L.; Reynolds, E.O.R. Relation between cerebral oxidative-metabolism following birth asphyxia, and neurodevelopmental outcome and brain growth at one year. Dev. Med. Child Neurol. 1992, 34, 285–295.
