This investigation presents a critical analysis of calcium aluminate cements (CAC), specifically associated with sustainability and environmental impact, and the potential of these cements to help solve certain worldwide problems. Areas of research include cements as recycling holding materials, sustainability, circular economy, production costs, and energy. Here summarizes the current trends, perspectives, and the main concerns regarding CAC. Detailed information about the materials and processes involved in CAC is also presented. First, a general search was made using the Carrot2 Workbench metasearch engine to identify possible thematic groups correlated with CAC, then a more in-depth and specialized search was done using the Scopus database.
- cements
- materials
- sustainability
- construction
- civil engineering
- phases
1. Introduction
| Mineral | Chemical Composition (wt%) | Tm (°C) | Density (g/cm3) |
Crystal Structure | Formation Enthalpy (kJ/mol) | |||
|---|---|---|---|---|---|---|---|---|
| CaO | Al2O3 | Fe2O3 | SiO2 | |||||
| C | 99.8 | - | - | - | 2570 | 3.32 | Cubic | - |
| C12A7 | 48.6 | 51.4 | - | - | 1405–1495 | 2.69 | Cubic | - |
| CA | 35.4 | 64.6 | - | - | 1600 | 2.98 | Monoclinic | −2323 |
| CA2 | 21.7 | 78.3 | - | - | 1750–1765 | 2.91 | Monoclinic | −4023 |
| C2S | 65.1 | - | - | 34.9 | 2066 | 3.27 | Monoclinic | - |
| C4AF | 46.2 | 20.9 | 32.9 | - | 1415 | 3.77 | Orthorhombic | - |
| C2AS | 40.9 | 37.2 | - | 21.9 | 1590 | 3.04 | Tetragonal | - |
| CA6 | 8.4 | 91.6 | - | - | 1830 | 3.38 | Hexagonal | - |
| Al2O3 | - | 99.8 | - | - | 2051 | 3.98 | Rhombohedral | |
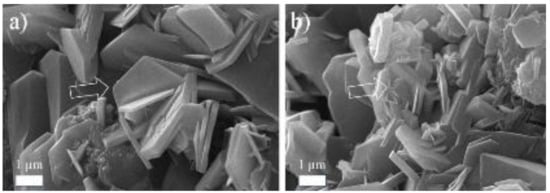
2. Analysis by Specific Area
2.1. Analysis by Environmental Area
| Article | Conference Paper | Review | Conference Review | Book Chapter | Production Interval (In Years) |
|
|---|---|---|---|---|---|---|
| Recycling | 37 | 7 | 1 | 4 | 3 | 1995–2022 |
| Production costs | 13 | 3 | 1 | 0 | 0 | 1995–2021 |
| Environmental Science | 3 | 2 | 0 | 0 | 0 | 2001–2020 |
| Additive manufacturing | 3 | 1 | 1 | 1 | 2 | 2010–2021 |
| CO2 generation | 4 | 1 | 0 | 0 | 0 | 2011–2021 |
| Sustainability | 10 | 4 | 1 | 1 | 1 | 2007–2021 |
| Circular economy | 4 | 0 | 0 | 0 | 0 | 2017–2021 |
| Life cycle assessment | 1 | 2 | 0 | 1 | 0 | 2014–2021 |
2.1.1. CAC + Recycling
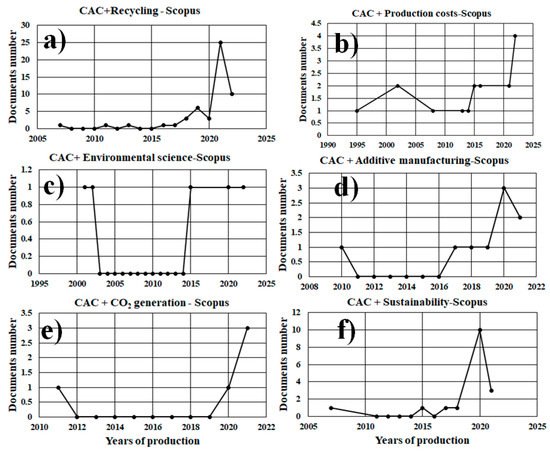
2.1.2. CAC + Production Costs
2.1.3. CAC + Environmental Science
2.1.4. CAC + Additive Manufacturing
2.1.5. CAC + CO2 Generation
2.1.6. CAC + Sustainability
2.1.7. CAC + Life Cycle Assessment
This entry is adapted from the peer-reviewed paper 10.3390/su14052751
References
- Abolhasani, A.; Samali, B.; Aslani, F. Physicochemical, mineralogical, and mechanical properties of calcium aluminate cement concrete exposed to elevated temperatures. Materials 2021, 14, 3855.
- Scrivener, K. Calcium aluminate. Adv. Concr. Technol. Set 2003, 1, 2.
- Abolhasani, A.; Samali, B.; Dehestani, M.; Libre, N.A. Effect of rice husk ash on mechanical properties, fracture energy, brittleness and aging of calcium aluminate cement concrete. Structures 2022, 36, 140–152.
- Zapata, J.F.; Gomez, M.; Colorado, H.A. Structure-property relation and Weibull analysis of calcium aluminate cement pastes. Mater. Charact. 2017, 134, 9–17.
- Zapata, J.F.; Gomez, M.; Colorado, H.A. High Temperature Cracking Damage of Calcium Aluminate Cements. In Proceedings of the TMS Annual Meeting & Exhibition; Springer: Berlin/Heidelberg, Germany, 2018; pp. 553–563.
- Zapata, J.F.; Gomezc, M.; Colorado, H.A. Characterization of two calcium aluminate cement pastes. In Advances in High Temperature Ceramic Matrix Composites and Materials for Sustainable Development; Wiley-American Ceramic Society: Hoboken, NJ, USA, 2017; pp. 491–503.
- Zapata, J.F.; Gomez, M.; Colorado, H.A. Calcium Aluminate Cements Subject to High Temperature. Adv. Mater. Sci. Environ. Energy Technol. VI 2017, 262, 97.
- Zapata, J.F.; Gomez, M.; Colorado, H.A. Cracking in Calcium Aluminate Cement Pastes Induced at Different Exposure Temperatures. J. Mater. Eng. Perform. 2019, 28, 7502–7513.
- Ukrainczyk, N.; Matusinovic, T.; Kurajica, S.; Zimmermann, B.; Sipusic, J. Dehydration of a layered double hydroxide—C2AH8. Thermochim. Acta 2007, 464, 7–15.
- Cardoso, F.A.; Innocentini, M.D.M.; Akiyoshi, M.M.; Pandolfelli, V.C. Effect of curing time on the properties of CAC bonded refractory castables. J. Eur. Ceram. Soc. 2004, 24, 2073–2078.
- Capmas, A.; Menetrier-Sorrentino, D.; Damidot, D. Effect of temperature on setting time of calcium aluminate cements. In Calcium Aluminate Cements; Taylor & Francis: New York, NY, USA, 1990; pp. 65–80.
- Bushnell-Watson, S.M.; Sharp, J.H. On the cause of the anomalous setting behaviour with respect to temperature of calcium aluminate cements. Cem. Concr. Res. 1990, 20, 677–686.
- Lee, W.E.; Vieira, W.; Zhang, S.; Ahari, K.G.; Sarpoolaky, H.; Parr, C. Castable refractory concretes. Int. Mater. Rev. 2001, 46, 145–167.
- Barnes, P.; Bensted, J. Structure and Performance of Cements; CRC Press: Boca Raton, FL, USA, 2014; ISBN 1482295016.
- Da Luz, A.P.; Braulio, M.d.A.L.; Pandolfelli, V.C. Refractory Castable Engineering; FIRE, Federation for International Refractory Research and Education: Baden-Baden, Germany, 2015; ISBN 3872640046.
- Mercury, J.M.R.; AZAa, A.; Turrillas, X.; Pena, P. Hidratación de los cementos de aluminatos de calcio (Parte I). Boletín Soc. Española Cerámica Vidr. 2003, 42, 269–276.
- Scrivener, K.L.; Capmas, A. Calcium aluminate cements. In Lea’s Chemistry of Cement and Concrete; Wiley: Hoboken, NJ, USA, 1998; pp. 713–782.
- Hewlett, P.; Liska, M. Lea’s Chemistry of Cement and Concrete; Butterworth-Heinemann: Oxford, UK, 2019; ISBN 978-0-08-100773-0.
- Midgley, H.G. Quantitative determination of phases in high alumina cement clinkers by X-ray diffraction. Cem. Concr. Res. 1976, 6, 217–223.
- Lothenbach, B.; Matschei, T.; Möschner, G.; Glasser, F.P. Thermodynamic modelling of the effect of temperature on the hydration and porosity of Portland cement. Cem. Concr. Res. 2008, 38, 1–18.
- Matschei, T.; Lothenbach, B.; Glasser, F.P. Thermodynamic properties of Portland cement hydrates in the system CaO–Al2O3–SiO2–CaSO4–CaCO3–H2O. Cem. Concr. Res. 2007, 37, 1379–1410.
- Scrivener, K.L.; Cabiron, J.-L.; Letourneux, R. High-performance concretes from calcium aluminate cements. Cem. Concr. Res. 1999, 29, 1215–1223.
- Bentsen, S. Effect of microsilica on conversion of high alumina cement. In Calcium Aluminate Cements; Chapman and Hall: London, UK, 1990; p. 294.
- Parker, K.M. Refractory calcium aluminate cements. Trans. Br. Ceram. Soc. 1982, 82, 35–42.
- Antonovič, V.; Kerienė, J.; Boris, R.; Aleknevičius, M. The effect of temperature on the formation of the hydrated calcium aluminate cement structure. Procedia Eng. 2013, 57, 99–106.
- Zapata, J.F.; Colorado, H.A.; Gomez, M.A. Effect of high temperature and additions of silica on the microstructure and properties of calcium aluminate cement pastes. J. Sustain. Cem. Mater. 2020, 1–27.
- Gartner, E. Industrially interesting approaches to “low-CO2” cements. Cem. Concr. Res. 2004, 34, 1489–1498.
- Bizzozero, J. Hydration and Dimensional Stability of Calcium Aluminate Cement Based Systems. Available online: https://infoscience.epfl.ch/record/202031 (accessed on 1 January 2022).
- Rahimi, M.; Esfahanian, M.; Moradi, M. Effect of reprocessing on shrinkage and mechanical properties of ABS and investigating the proper blend of virgin and recycled ABS in injection molding. J. Mater. Process. Technol. 2014, 214, 2359–2365.
- Prendergast, M.E.; Burdick, J.A. Recent advances in enabling technologies in 3D printing for precision medicine. Adv. Mater. 2020, 32, 1902516.
- Browne, M.P.; Redondo, E.; Pumera, M. 3D printing for electrochemical energy applications. Chem. Rev. 2020, 120, 2783–2810.
- Distler, T.; Boccaccini, A.R. 3D printing of electrically conductive hydrogels for tissue engineering and biosensors–A review. Acta Biomater. 2020, 101, 1–13.
- He, P.; Zhang, B.; Lu, J.-X.; Poon, C.S. ASR expansion of alkali-activated cement glass aggregate mortars. Constr. Build. Mater. 2020, 261, 119925.
- Zhang, B.; He, P.; Poon, C.S. Optimizing the use of recycled glass materials in alkali activated cement (AAC) based mortars. J. Clean. Prod. 2020, 255, 120228.
- Panpa, W.; Jinawath, S.; Kashima, D.P. Ag2O-Ag/CAC/SiO2 composite for visible light photocatalytic degradation of cumene hydroperoxide in water. J. Mater. Res. Technol. 2019, 8, 5180–5193.
- Hossain, S.K.S.; Roy, P.K. Development of waste derived nano-lakargiite bonded high alumina refractory castable for high temperature applications. Ceram. Int. 2019, 45, 16202–16213.
- Al-musawi, H.; Figueiredo, F.P.; Guadagnini, M.; Pilakoutas, K. Shrinkage properties of plain and recycled steel–fibre-reinforced rapid hardening mortars for repairs. Constr. Build. Mater. 2019, 197, 369–384.
- Al-musawi, H.; Figueiredo, F.P.; Bernal, S.A.; Guadagnini, M.; Pilakoutas, K. Performance of rapid hardening recycled clean steel fibre materials. Constr. Build. Mater. 2019, 195, 483–496.
- Yıldırım, S.T.; Baynal, K.; Fidan, O. Internal Curing and Temperature Effect on Lightweight and Heat Insulated Mortar with Recycled Concrete Aggregate. Acta Phys. Pol. A 2019, 135, 865–869.
- Kulu, P.; Goljandin, D.; Külaviir, J.; Hain, T.; Kivisto, M. Recycling of Niobium Slag by Disintegrator Milling. In Key Engineering Materials; Trans Tech Publications: Baech, Switzerland, 2019; Volume 799, pp. 97–102.
- Ogrodnik, P.; Szulej, J.; Franus, W. The Wastes of Sanitary Ceramics as Recycling Aggregate to Special Concretes. Materials 2018, 11, 1275.
- Nematzadeh, M.; Dashti, J.; Ganjavi, B. Optimizing compressive behavior of concrete containing fine recycled refractory brick aggregate together with calcium aluminate cement and polyvinyl alcohol fibers exposed to acidic environment. Constr. Build. Mater. 2018, 164, 837–849.
- Nematzadeh, M.; Baradaran-Nasiri, A. Residual properties of concrete containing recycled refractory brick aggregate at elevated temperatures. J. Mater. Civ. Eng. 2018, 30, 4017255.
- Baradaran-Nasiri, A.; Nematzadeh, M. The effect of elevated temperatures on the mechanical properties of concrete with fine recycled refractory brick aggregate and aluminate cement. Constr. Build. Mater. 2017, 147, 865–875.
- Stonys, R.; Kuznetsov, D.; Krasnikovs, A.; Škamat, J.; Baltakys, K.; Antonovič, V.; Černašėjus, O. Reuse of ultrafine mineral wool production waste in the manufacture of refractory concrete. J. Environ. Manag. 2016, 176, 149–156.
- Navarro-Blasco, Í.; Fernández, J.M.; Duran, A.; Sirera, R.; Alvarez, J.I. A novel use of calcium aluminate cements for recycling waste foundry sand (WFS). Constr. Build. Mater. 2013, 48, 218–228.
- Fernández, L.J.; Ferrer, R.; Aponte, D.F.; Fernandez, P. Recycling silicon solar cell waste in cement-based systems. Sol. Energy Mater. Sol. cells 2011, 95, 1701–1706.
- Chen, L.; Wang, Y.-S.; Wang, L.; Zhang, Y.; Li, J.; Tong, L.; Hu, Q.; Dai, J.-G.; Tsang, D.C.W. Stabilisation/solidification of municipal solid waste incineration fly ash by phosphate-enhanced calcium aluminate cement. J. Hazard. Mater. 2021, 408, 124404.
- Erans, M.; Jeremias, M.; Zheng, L.; Yao, J.G.; Blamey, J.; Manovic, V.; Fennell, P.S.; Anthony, E.J. Pilot testing of enhanced sorbents for calcium looping with cement production. Appl. Energy 2018, 225, 392–401.
- Xu, L.; Wang, P.; Wu, G.; Li, N. Effect of calcium aluminate cement on hydration properties and microstructure of portland cement. Materials 2015, 13, 4000.
- Akiti, T.T., Jr.; Constant, K.P.; Doraiswamy, L.K.; Wheelock, T.D. An improved core-in-shell sorbent for desulfurizing hot coal gas. Adv. Environ. Res. 2002, 6, 419–428.
- Akiti, T.T., Jr.; Constant, K.P.; Doraiswamy, L.K.; Wheelock, T.D. Development of an advanced calcium-based sorbent for desulfurizing hot coal gas. Adv. Environ. Res. 2001, 5, 31–38.
- Lv, H.; Xie, M.; Shi, L.; Zhao, H.; Wu, Z.; Li, L.; Li, R.; Liu, F. A novel green process for the synthesis of high-whiteness and ultrafine aluminum hydroxide powder from secondary aluminum dross. Ceram. Int. 2022, 48, 953–962.
- Shakor, P.; Nejadi, S.; Paul, G.; Sanjayan, J. A novel methodology of powder-based cementitious materials in 3D inkjet printing for construction applications. In Proceedings of the Sixth International Conference on Durability of Concrete Structures (ICDCS 2018), Leeds, UK, 18–20 July 2018.
- Shakor, P.; Sanjayan, J.; Nazari, A.; Nejadi, S. Modified 3D printed powder to cement-based material and mechanical properties of cement scaffold used in 3D printing. Constr. Build. Mater. 2017, 138, 398–409.
- Antonovič, V.; Pundiene, I.; Stonys, R.; Česniene, J.; Keriene, J. A review of the possible applications of nanotechnology in refractory concrete. J. Civ. Eng. Manag. 2010, 16, 595–602.
- Das, A.; Reiter, L.; Mantellato, S.; Flatt, R.J. Blended calcium aluminate cements for digital fabrication with concrete. In Proceedings of the 5th International Conference on Calcium Aluminates (2022), Cambridge, UK, 1–3 June 2020; ETH Zurich-Institute of Building Materials: Zurich, Switzerland, 2022.
- Bharati, S.; Sah, R.; Sambandam, M. Green Castable Using Steelmaking Slags: A Sustainable Product for Refractory Applications. J. Sustain. Metall. 2020, 6, 113–120.
- Giroudon, M.; Lavigne, M.P.; Patapy, C.; Bertron, A. Biodeterioration mechanisms and kinetics of SCM and aluminate based cements and AAM in the liquid phase of an anaerobic digestion. MATEC Web Conf. 2018, 199, 2003.
- Moradllo, M.K.; Ley, M.T. Comparing ion diffusion in alternative cementitious materials in real time by using non-destructive X-ray imaging. Cem. Concr. Compos. 2017, 82, 67–79.
- Boden, T.; Andres, B.; Marland, G. Global, Regional, and National Fossil-Fuel CO2 Emissions (1751–2010); OSTI. GOV: Oak Ridge, TN, USA, 2013.
- Burris, L.E.; Alapati, P.; Moser, R.D.; Ley, M.T.; Berke, N.; Kurtis, K.E. Alternative cementitious materials: Challenges and opportunities. In Proceedings of the International Workshop on Durability and Sustainability of Concrete Structures, Bologna, Italy, 1–3 October 2015.
- Henry-Lanier, E.; Szepizdyn, M.; Parr, C. Optimisation of the Environmental Footprint of Calcium-Aluminate-Cement Containing Castables. Refract. Worldforum 2015, 8, 81–86.
