Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Plant Sciences
RNA/DNA difference (RDD) is a post-transcriptional RNA modification to enrich genetic information, widely involved in regulating diverse biological processes in eukaryotes. RDDs in the wheat nuclear genome, especially those associated with drought response or tolerance, were not well studied up to now.
- drought
- RNA/DNA difference
- wheat
1. Introduction
Wheat (Triticum aestivum L.) is one of the most important cereal crops all over the world, providing the staple food source for about 30% of the global population and occupying approximately 20% of the world’s cultivated lands [1].
RNA/DNA differences (RDDs) are phenomena of base insertion, deletion or modification, occurring when DNA is transcribed into RNA [2]. This widespread post-transcriptional modification mechanism contributes significantly to the diversity and plasticity of cellular RNA signatures, increasing proteomic diversity by modifying the sequence of primary transcripts that do not operate completely or partially [3][4]. Together with alternative splicing, RDD provides a crucial way to enrich genetic information and diversify transcripts, playing an important role in regulating growth, development and stress response in eukaryotes [5]. ‘Substitution’ by simple base modification is the most common type of RDD, and is widely identified in plant organelle and higher eukaryotic nuclei genomes [6][7] as well as some viral sequences [8]. In mammals, the common type of RDD is A-to-I (G) (adenosine to inosine, guanosine), which is mainly catalyzed by double-stranded RNA-specific ADAR family proteins [9]. It is also called as A-to-G editing because the inosine in RNA is interpreted as guanosine (G) by the translation mechanism [10]. At the same time, A-to-I transformation independent of ADAR enzyme was also found in fungi [9]. However, ADAR-like enzymes have not been found in plants [11]. Another type, cytidine to uridine (C-to-U), is found to be catalyzed by APOBECs activation in humans [12], while the mechanism of the other remaining 10 modification types is still unclear [13][14]. In plants, RNA/DNA difference is generally found in organellar transcripts, and mainly regulated by the specific pentatripeptide repeat proteins encoded by the nuclear genome that deaminate cytidine to uridine [4][15][16]. There are two main types of PPR proteins: P and PLS [17]. The PLS group seems to be the most relevant class for RNA editing in plants with a conserved extended domain at the C-terminal and DYW motif [18][19][20]. The DYW motif encodes the active center of cytidine deaminase, which may be responsible for C-to-U modification [18].
2. Researches and Findings
2.1. Identification of RNA/DNA Differences Based on RNA-Seq Data in Wheat
Based on the approach described in the Methods section, a total of 15,339, 10,821 and 12,502 RDDs were found in the control samples of crown, leaf and root respectively, while 15,167, 10,960, 12,429 RDDs were identified in drought-stressed samples of the crown, leaf and root, respectively (Figure 1A,B). It seems that the CK sample displayed slightly higher RDDs compared to the drought-treated sample in all of the 3 tissues. Meanwhile, for both the CK and drought condition, leaves had the lowest RDDs. These results indicated that RDDs were widely found in wheat nuclear genome. Furthermore, 21,782 unique RDDs were obtained through combining these identified RDDs, of which 8675 appeared in protein-coding genes. Among them, 4205 genes had one RDD, followed by 1794, 1068 and 588 genes with 2, 3 and 4 RDDs, and 1020 genes with more than 5 RDDs, respectively. Overall, 15,623, 11,216, 12,809 RDDs corresponding to 6618, 4959 and 5331 genes were found in crown, leaves and root respectively, of which 6645 RDDs in 3151 genes were commonly found in all of the three tissues (Figure 1C). For RNA/DNA difference type, all of the 12 conversion types were found, of which the conversion between C and T accounted for 24.19% of all sites, and conversion between A and G accounted for 24.31% respectively, which were the two canonical RNA/DNA difference types [21]. Additionally, C to G (7.18%) and G to C (7.09%), A to C (6.99%), T to G (6.86%), T to A (6.67%), A to T (6.14%), and C to A (5.40%) as well as G to T (5.17%) were also identified (Figure 1D). Gene annotation showed that 12,325, 2770, 1250 and 604 RDDs were located in the CDS, intron, 5′UTR and 3′UTR regions, respectively. Furthermore, 4513 and 3948 RDDs were annotated as missense variants and synonymous variants, accounting for 20.72% and 18.13% of RDDs, respectively, of which 189 RDDs could result in the premature stop or loss of the start/termination codons (Figure 1E).
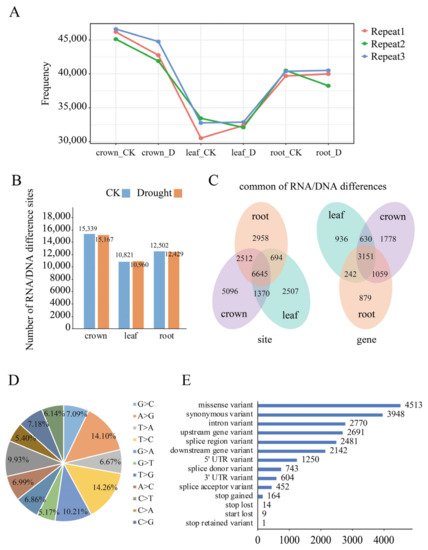
Figure 1. Characterization of RNA/DNA differences in wheat nuclear genome using RNA-Seq data. (A) The number of variations identified in three biological replicates of control and drought conditions in the crown, leaf and root, respectively; (B) The numbers of unique RNA/DNA differences identified in crown, leaf and root, respectively; (C) The shared and unique RDD sites (Left) and RDD-containing genes (Right) found in crown, leaf and root tissues; (D) The variation types of the identified unique RDDs; (E) The frequency distribution of RDDs in the transcription regions. The y-axis represents different types of gene regions, and the x-axis represents the abundance of RDDs.
2.2. Identification of RDDs Associated with Drought Response
Based on the above identified RDDs, the potential RDDs associated with drought stress through comparing the CK and stressed samples combined with IGV viewing. In total, 265 drought-responsive RDDs were obtained, of which 78, 97 and 90 RDDs were found in crown, leaves and root, respectively. Surprisingly, drought-responsive RDD events displayed strong tissue specificity that none was shared by the three tissues. Among them, 76, 100 and 89 RDDs were located on subgenome A, B and D, corresponding to 54, 69 and 53 protein-coding genes, respectively (Figure 2A). Furthermore, 186 and 79 drought-responsive RDDs were located in CDS and UTR regions respectively, of which, 75 RDDs could cause missense variants (Figure 2B). These RDDs that could cause amino acid changes might play an important regulatory role in coping with drought stress response and tolerance. For difference type, there also all 12 types were found. Similar to the overall RDDs, the two canonical types conversion between C and T together with conversion between A and G were also the most abundant types (Figure 2C), similar to wild grapevine [22][23]. It is obvious that base transition events were higher than base transversion in these RDDs, although transversions should have twice higher occurrence frequency (transition/transversion ratio was 1.55), suggesting that these RDDs were not randomly occurring and related to the specific transcription regulation mechanism underlying the RND/DNA differences resulting from drought stress.
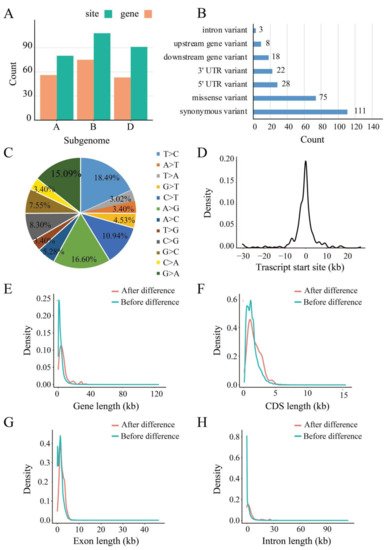
Figure 2. Characterization of the drought-responsive RDDs. (A) The numbers of drought-responsive RDD and related-genes in A, B, D subgenome, respectively; (B) The distribution of drought-responsive RDDs in the transcription regions. The y-axis represents different types of regions, and the x-axis represents the abundance of RNA/DNA difference sites; (C) The variation types of the identified drought-responsive RDDs; (D) The distance of the distribution of drought-responsive RDD site and the TSS (transcription start site) in the RDD-containing genes; (E–H) Comparison of gene length, CDS length, exon length, and intron length of gene without RDD and with RDD.
Results showed that they were mainly located about 10kb upstream or downstream of the translational start site (TSS) of their corresponding genes (Figure 2D); it is suggested that RDDs may impact on the expression of the corresponding genes. Compared to the genes that did not contain RDDs, the genes containing RDDs have significantly longer gene, exon, CDS and intron size (Figure 2E–H). Furthermore, the difference occurrence efficiency was also calculated based on the ratio of the number of difference reads to the total reads for each RDD site (Figure 3A). Results found that these RDDs under the drought condition displayed significantly higher difference efficiency than that of CK. The efficiency value of the drought-responsive RDDs ranged from 0.07 to 0.83, with most showing 0.1 to 0.2 (108 sites), followed by 0.2 to 0.3 (55 sites), 0.3 to 0.4 (41 sites), 0.4 to 0.5 (22 sites), 0.5 to 0.6 (13 sites), 0.6 to 0.7 (10 sites), 0 to 0.1 (9 sites), 0.7 to 0.8 (4 sites) and 0.8 to 0.9 (3 sites). The efficiency density of the three tissues is skewed to the left (Figure 3B), suggesting that all drought-responsive RDDs had relatively low difference efficiency in all tissues. Total RNA and DNA was extracted from the leaves. Results showed that the T to C mutation was not found in genomic DNA or the cDNA of CK samples, but appeared in the cDNA of drought stressed samples (Figure 3C), indicating that it is an actual drought-responsive RDD site.
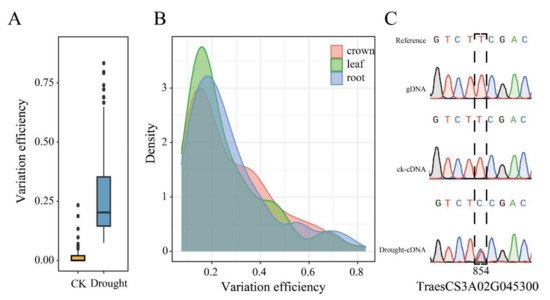
Figure 3. Variation efficiency and validation of these identified RDDs. (A) Comparison of the variation efficiency of RDDs between CK and drought conditions; (B) the distribution of variation efficiency in 3 different tissues; (C) Validation of the randomly selected drought-responsive RDD in TraesCS3A02G045300 through RT-PCR combined with Sanger sequencing.
2.3. Effects of RDDs on Gene Expression
To explore the relationship between RDDs and gene expression, we further investigated the expression level of the genes containing drought-responsive RDDs under CK and drought conditions. In total, 35 unique genes were found to display differential expression patterns (Figure 4A), suggesting they might play a role in the regulation process of drought response. In detail, 11 (6 down-regulated and 5 up-regulated), 15 (9 down-regulated and 6 up-regulated) and 9 (8 down-regulated and 1 up-regulated) differential RDD-containing genes were found in root, leaf and crown, respectively. Among them, TraesCS2B02G038700 is annotated to encode a Chalcone synthase. Chalcone synthase is the key enzyme controlling flavonoid biosynthesis, which has been proven to monitor the drought stress tolerance [24]. TraesCS2B02G079100 is the orthology of OsRBCS, which is a drought stress related marker gene [25]. These results indicated that RDDs in these drought related genes might mediate their expression to regulate the drought response process. Furthermore, functional enrichment found that they are significantly enriched in stress response related terms, such as ‘defense response’, ‘response to oxidative stress’ and ‘response to desiccation’. In addition, they are also enriched in terms related to the structure of DNA or RNA, including ‘mRNA binding’, ‘DNA binding’. In KEGG pathway, they are enriched in the biosynthesis of secondary metabolites, flavonoids biosynthesis, and zeatin biosynthesis, all of which were also related to the drought response in plants (Figure 4B).
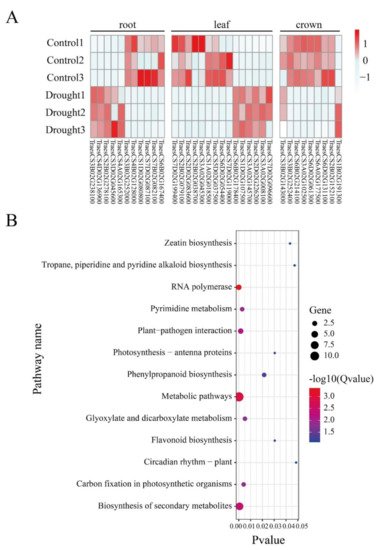
Figure 4. Differential expression and functional enrichment of the genes occurring in drought-responsive RDD. (A) The differential expression of RDD-containing genes between CK and drought conditions in roots, leaf and crown tissues; (B) KEGG enrichment analysis of these differentially expressed RDD-containing genes.
2.4. Effect of RDDs on RNA Secondary Structure and MiRNA Targeting
Secondary structural transformation, as a binary switch activated by cellular signals, is a general mechanism in gene regulation networks [26]. The secondary structure changes of these genes will impact on achieving their function, indicating that the RDDs might be involved in regulating drought response through influencing the structural stability of the drought-related genes. Further study on the specific role of RDD in regulating RNA secondary structure will contribute to better understanding of the genetic basis of crop drought resistance.
miRNA is a class of small non-coding RNAs that often bind to complementary sequences of the mRNA targets to silence or weaken their expression [27]. RDD generally caused variations in mRNA sequence, which could make the transcripts gain or lose miRNA binding [28]. To understand the effects of drought responsive RDDs on miRNA targeting, we further investigated miRNA-mRNA targeting pairs using the genes with or without RDD mutations as target genes. Results showed that there were 160 genes that were potentially targeted by 81 unique high-confidence mature miRNAs of wheat, containing 236 RDDs (Table S5), of which 3 RDDs occurring in 3 genes were detected to result in the changing of miRNA targets compared to the initial genes without RDD. We found two RDD sites (chr2A_21252947: A to T; chr2A_21252961: A to C) occurring in TraesCS2A02G053100 (Figure 5A): the T to A site changed the amino acid from Phe to Tyr (Figure 5B), and the T to G site made it lose the binding of tae-miR9657b-5p (Figure 5C). Meanwhile, the RNA secondary structure of this gene was also changed by the RDD sites (Figure 5D), that the MFE value changed from −657.40 to −659.40 kcal/mol. Furthermore, the difference efficiency of T to G in TraesCS2A02G053100 is 0.18 under the drought condition while not occurring in CK with the variation efficiency of 0 (Figure 5E). The expression level of this gene was also slightly higher under the drought condition compared to CK (Figure 5F). These results indicated that RDD could precisely regulate the expression level of the target genes through mediating miRNA targeting with variable variation efficiency. In addition, orthologs analysis found that TraesCS2A02G053100 is the ortholog of OsRLCK. OsRLCK is reported as the key candidate associated with QTL for drought stress related traits [29], suggesting that TraesCS2A02G053100 could also have potential function in response to drought stress. The RDD occurring in this gene might play an indispensable role in regulating the sophisticated process of drought response that this gene was involved in.
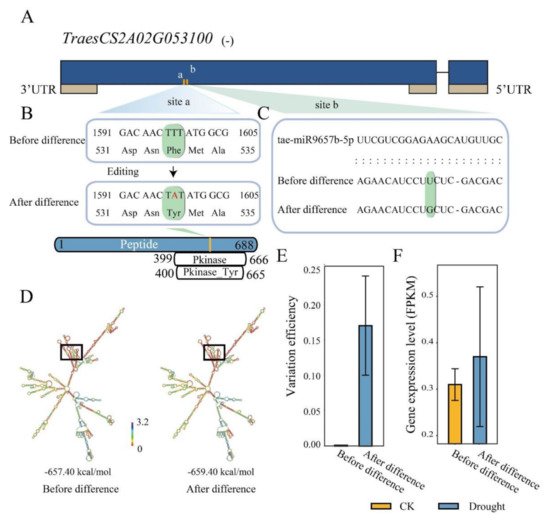
Figure 5. RNA/DNA difference functions on the miRNA targeting in TraesCS2A02G053100. (A) Two RNA/DNA difference sites (a, b) were found in TraesCS2A02G053100, which is annotated in the reverse chain of the genome; (B) The RDD site A to T (site a) caused amino acid Phe changed into Tyr; (C) The RDD site A to C (site b) leads to the loss of miR9657-5p targeting; (D) Change of the RNA 2D structure before and after RDD variations; (E) Variation efficiency of the A to C site (site b); (F) Expression levels of TraesCS2A02G053100 before and after variation.
2.5. Effects of RDDs on Protein Conserved Domain
RNA/DNA difference is a post-transcriptional modification mechanism to increase proteomic diversity by modifying protein composition, protein structure and binding ability [30]. To understand the effect of drought-responsive RDD on the encoded protein structure, we investigated and compared the conserved domain organization of the genes with missense variant and stop gained due to RDD before and after RDD occurred. It is reported that AtABA2 encoded xanthin dehydrogenase, which is a key enzyme for abscisic acid (ABA) biosynthesis, to play the crucial role in regulating ABA levels in Arabidopsis [31][32]. The other gene is TraesCS7A02G162400, which is the orthology of G-protein coupled receptor 1 (GCR1) in Arabidopsis. A RDD site T to C (chr7A_118456566) in TraesCS7A02G162400 resulted in loss of the transmembrane receptor domain although the slime mold cyclic AMP receptor domain was present (Figure 6A,B). The expression level of this gene was also down-regulated after RDD occurred (Figure 6C), and the protein three-dimensional (3D) structure predictions also displayed differences before and after RDD (Figure 6D). These results indicated the drought-responsive RDD could also impact on the protein domain of the drought-related genes and act as the regulator in the biological process of drought response and tolerance.
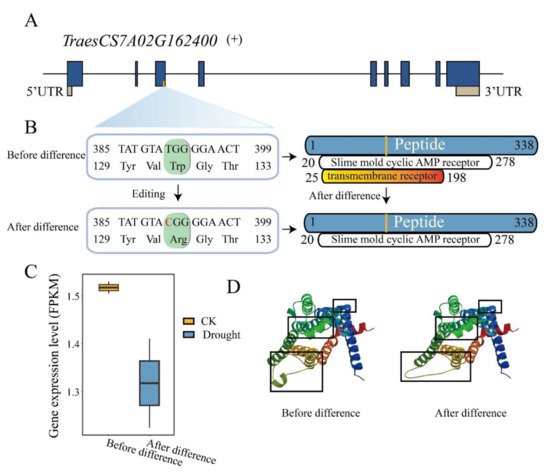
Figure 6. RNA/DNA difference impacts on the protein conserved domains and 3D structure in TraesCS7A02G162400. (A,B) A T to C variation was identified in the third exon of TraesCS7A02G162400, which caused Trp to Arg change, and lost a protein conserved domain; (C) Expression levels of TraesCS7A02G162400 before and after variation; (D) Changes of protein 3D structure before and after difference.
This entry is adapted from the peer-reviewed paper 10.3390/ijms23031405
References
- Shiferaw, B.; Smale, M.; Braun, H.J.; Duveiller, E.; Reynolds, M.; Muricho, G. Crops that feed the world 10. Past successes and future challenges to the role played by wheat in global food security. Food Secur. 2013, 5, 291–317.
- Peng, Z.; Cheng, Y.; Tan, B.C.M.; Kang, L.; Tian, Z.; Zhu, Y.; Zhang, W.; Liang, Y.; Hu, X.; Tan, X.; et al. Comprehensive analysis of RNA-Seq data reveals extensive RNA editing in a human transcriptome. Nat. Biotechnol. 2012, 30, 253–260.
- Nishikura, K. A-to-I editing of coding and non-coding RNAs by ADARs. Nat. Rev. Mol. Cell Biol. 2016, 17, 83–96.
- Gray, M. Diversity and evolution of mitochondrial RNA editing systems. IUBMB Life 2003, 55, 227–233.
- Bian, Z.; Ni, Y.; Xu, J.R.; Liu, H. A-to-I mRNA editing in fungi: Occurrence, function, and evolution. Cell. Mol. Life Sci. 2019, 76, 329–340.
- Qiu, Y.L.; Li, L.; Wang, B.; Chen, Z.; Dombrovska, O.; Lee, J.; Kent, L.; Li, R.; Jobson, R.W.; Hendry, T.A.; et al. A nonflowering land plant phylogeny inferred from nucleotide sequences of seven chloroplast, mitochondrial, and nuclear genes. Int. J. Plant Sci. 2007, 168, 691–708.
- Takenaka, M.; Verbitskiy, D.; van der Merwe, J.A.; Zehrmann, A.; Brennicke, A. The process of RNA editing in plant mitochondria. Mitochondrion 2008, 8, 35–46.
- Wang, L.; Sun, Y.; Song, X.; Wang, Z.; Zhang, Y.; Zhao, Y.; Peng, X.; Zhang, X.; Li, C.; Gao, C.J.; et al. Hepatitis B virus evades immune recognition via RNA adenosine deaminase ADAR1-mediated viral RNA editing in hepatocytes. Cell. Mol. Immunol. 2021, 18, 1871–1882.
- Savva, Y.A.; Rieder, L.E.; Reenan, R.A. The ADAR protein family. Genome Biol. 2012, 13, 252.
- Nishikura, K. Editor meets silencer: Crosstalk between RNA editing and RNA interference. Nat. Rev. Mol. Cell Biol. 2006, 7, 919–931.
- Jin, Y.; Zhang, W.; Li, Q. Origins and evolution of ADAR-mediated RNA editing. IUBMB Life 2009, 61, 572–578.
- Salter, J.D.; Bennett, R.P.; Smith, H.C. The APOBEC protein family: United by structure, divergent in function. Trends Biochem. Sci. 2016, 41, 578–594.
- Li, M.; Wang, I.X.; Li, Y.; Bruzel, A.; Richards, A.L.; Toung, J.M.; Cheung, V.G. Widespread RNA and DNA sequence differences in the human transcriptome. Science 2011, 333, 53–58.
- Liscovitch-Brauer, N.; Alon, S.; Porath, H.T.; Elstein, B.; Unger, R.; Ziv, T.; Admon, A.; Levanon, E.Y.; Rosenthal, J.J.; Eisenberg, E. Trade-off between transcriptome plasticity and genome evolution in cephalopods. Cell 2017, 169, 191–202.
- Qulsum, U.; Azad, M.T.A.; Tsukahara, T. Analysis of tissue-specific RNA editing events of genes involved in RNA editing in Arabidopsis thaliana. J. Plant Biol. 2019, 62, 351–358.
- Small, I.D.; Schallenberg-Rüdinger, M.; Takenaka, M.; Mireau, H.; Ostersetzer-Biran, O. Plant organellar RNA editing: What 30 years of research has revealed. Plant J. 2020, 101, 1040–1056.
- Wang, X.; An, Y.; Xu, P.; Xiao, J. Functioning of PPR Proteins in organelle RNA metabolism and chloroplast biogenesis. Front. Plant Sci. 2021, 12, 1.
- Liu, X.Y.; Jiang, R.C.; Wang, Y.; Tang, J.J.; Sun, F.; Yang, Y.Z.; Tan, B.C. ZmPPR26, a DYW-type pentatricopeptide repeat protein, is required for C-to-U RNA editing at atpA-1148 in maize chloroplasts. J. Exp. Bot. 2021, 72, 4809–4821.
- Ichinose, M.; Sugita, M. The DYW domains of pentatricopeptide repeat RNA editing factors contribute to discriminate target and non-target editing sites. Plant Cell Physiol. 2018, 59, 1652–1659.
- Malbert, B.; Burger, M.; Lopez-Obando, M.; Baudry, K.; Launay-Avon, A.; Härtel, B.; Verbitskiy, D.; Jörg, A.; Berthomé, R.; Lurin, C.; et al. The Analysis of the Editing Defects in the dyw2 Mutant Provides New Clues for the Prediction of RNA Targets of Arabidopsis E+-Class PPR Proteins. Plants 2020, 9, 280.
- Yang, G.; Pan, Y.; Zhang, R.; Huang, J.; Pan, W.; Cui, L.; Song, W.; Nie, X. Genome-wide identification and characterization of DNA/RNA differences associated with Fusarium graminearum infection in wheat. bioRxiv 2021.
- Ramos, M.J.; Faísca-Silva, D.; Coito, J.L.; Cunha, J.; Silva, H.G.; Viegas, W.; Costa, M.M.R.; Amâncio, S.; Rocheta, M. RNA editing in inflorescences of wild grapevine unveils association to sex and development. bioRxiv 2020.
- Ichinose, M.; Sugita, M. RNA editing and its molecular mechanism in plant organelles. Genes 2017, 8, 5.
- Hu, B.; Yao, H.; Gao, Y.; Wang, R.; Jin, L. Overexpression of chalcone synthase gene improves flavonoid accumulation and drought tolerance in tobacco. Preprints 2019.
- Lee, D.K.; Jung, H.; Jang, G.; Jeong, J.S.; Kim, Y.S.; Ha, S.H.; Choi, Y.D.; Kim, J.K. Overexpression of the OsERF71 Transcription Factor Alters Rice Root Structure and Drought Resistance. Plant Physiol. 2016, 172, 575–588.
- Dethoff, E.A.; Chugh, J.; Mustoe, A.M.; Al-Hashimi, H.M. Functional complexity and regulation through RNA dynamics. Nature 2012, 482, 322–330.
- Meng, Y.; Chen, D.; Jin, Y.; Mao, C.; Wu, P.; Chen, M. RNA editing of nuclear transcripts in Arabidopsis thaliana. BMC Genom. 2010, 11 (Suppl. S4), S12.
- Picardi, E.; Horner, D.S.; Chiara, M.; Schiavon, R.; Valle, G.; Pesole, G. Large-scale detection and analysis of RNA editing in grape mtDNA by RNA deep-sequencing. Nucleic Acids Res. 2010, 38, 4755–4767.
- Vij, S.; Giri, J.; Dansana, P.K.; Kapoor, S.; Tyagi, A.K. The receptor-like cytoplasmic kinase (OsRLCK) gene family in rice: Organization, phylogenetic relationship, and expression during development and stress. Mol. Plant 2008, 1, 732–750.
- Gott, J.M. Expanding genome capacity via RNA editing. Comptes Rendus Biol. 2003, 326, 901–908.
- González-Guzmán, M.; Apostolova, N.; Bellés, J.M.; Barrero, J.M.; Piqueras, P.; Ponce, M.R.; Micol, J.L.; Serrano, R.; Rodríguez, P.L. The short-chain alcohol dehydrogenase ABA2 catalyzes the conversion of xanthoxin to abscisic aldehyde. Plant Cell 2002, 14, 1833–1846.
- Endo, A.; Nelson, K.M.; Thoms, K.; Abrams, S.R.; Nambara, E.; Sato, Y.J. Functional characterization of xanthoxin dehydrogenase in rice. J. Plant Physiol. 2014, 171, 1231–1240.
This entry is offline, you can click here to edit this entry!
