Pathogens, especially drug-resistant pathogens caused by the abuse of antibiotics, have become a major threat to human health and public health safety. The exploitation and application of new antibacterial agents is extremely urgent. As a natural biopolymer, cellulose has recently attracted much attention due to its excellent hydrophilicity, economy, biocompatibility, and biodegradability. In particular, the preparation of cellulose-based hydrogels with excellent structure and properties from cellulose and its derivatives has received increasing attention thanks to the existence of abundant hydrophilic functional groups (such as hydroxyl, carboxy, and aldehyde groups) within cellulose and its derivatives. The cellulose-based hydrogels have broad application prospects in antibacterial-related biomedical fields.
- hydrogels
- antibacterial
- cellulose
1. Cellulose-Based Antibacterial Hydrogels Loaded with Metal Nanoparticles
2. Cellulose-Based Antibacterial Hydrogels Loaded with Metal Oxide Nanoparticles
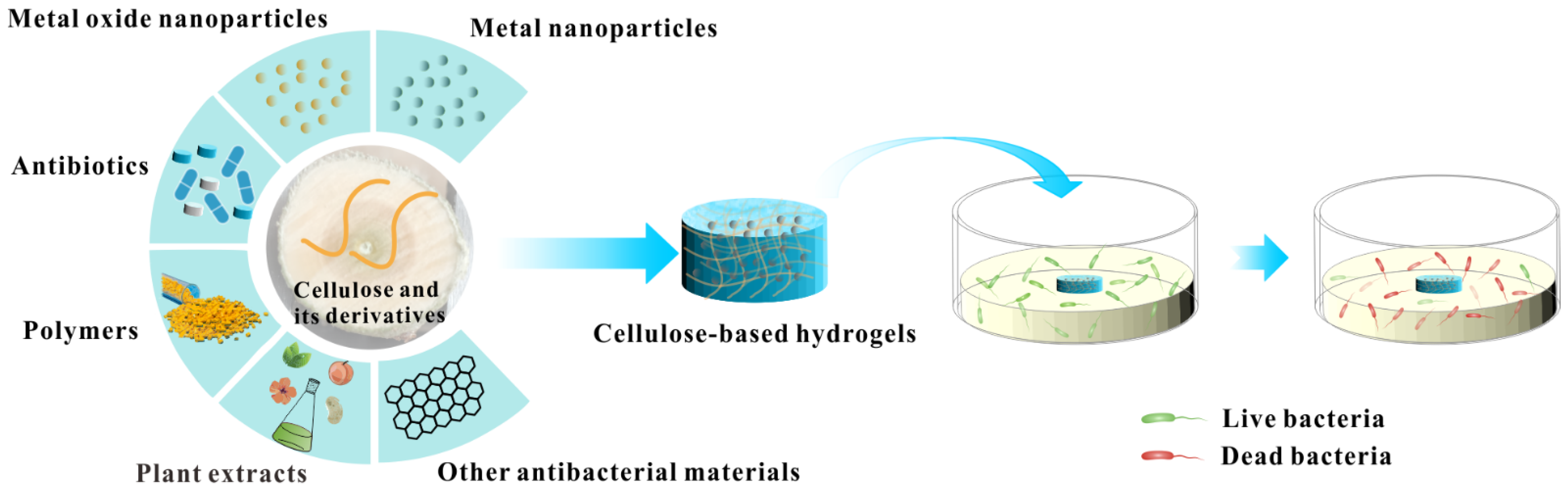
3. Cellulose-Based Antibacterial Hydrogels Loaded with Antibiotics
| Antibacterial Agents | Advantages | Disadvantages | Typical Applications | References |
|---|---|---|---|---|
| Metal or oxidized metal nanoparticles | Broad-spectrum and long-term antibacterial, low bacterial resistance | Tend to agglomerate, certain cytotoxicity and environmental toxicity | Wound dressing Self-healing artificial skin |
[12][19][48][49][50][51][52] |
| Antibiotics | Specific and efficient antibacterial, ideal biocompatibility | Bacterial resistance and short-term antibacterial activity, prone to degradation and instability | Clinical antibacterial Wound healing |
[35][37][53][54][55][56] |
| Polymers | Biodegradable and nontoxic, high modifiability and biocompatibility | Poor permeability | Antibacterial food packaging Treatment of periodontitis |
[47][57][58][59][60][61] |
| Plant extracts | Rich resources, environmentally friendly, anti-drug resistant bacteria | Difficult to extract and enrich | Biomedicine Treatment of chronic infection |
[62][63][64][65][66][67] |
This entry is adapted from the peer-reviewed paper 10.3390/polym14040769
References
- Tavakolian, M.; Munguia-Lopez, J.G.; Valiei, A.; Islam, M.S.; Kinsella, J.M.; Tufenkji, N.; van de Ven, T.G.M. Highly absorbent antibacterial and biofilm-disrupting hydrogels from cellulose for wound dressing applications. ACS Appl. Mater. Interfaces 2020, 12, 39991–40001.
- Oyarzun-Ampuero, F.; Vidal, A.; Concha, M.; Morales, J.; Orellana, S.; Moreno-Villoslada, I. Nanoparticles for the treatment of wounds. Curr. Pharm. Des. 2015, 21, 4329–4341.
- Liang, D.; Lu, Z.; Yang, H.; Gao, J.; Chen, R. Novel asymmetric wettable AgNPs/chitosan wound dressing: In vitro and in vivo evaluation. ACS Appl. Mater. Interfaces 2016, 8, 3958–3968.
- Mokhena, T.C.; Luyt, A.S. Electrospun alginate nanofibres impregnated with silver nanoparticles: Preparation, morphology and antibacterial properties. Carbohydr. Polym. 2017, 165, 304–312.
- Shao, W.; Wu, J.; Wang, S.; Huang, M.; Liu, X.; Zhang, R. Construction of silver sulfadiazine loaded chitosan composite sponges as potential wound dressings. Carbohydr. Polym. 2017, 157, 1963–1970.
- Bao, Y.; He, J.; Song, K.; Guo, J.; Zhou, X.; Liu, S. Plant-extract-mediated synthesis of metal nanoparticles. J. Chem. 2021, 2021, 6562687.
- Celebioglu, A.; Aytac, Z.; Umu, O.C.O.; Dana, A.; Tekinay, T.; Uyar, T. One-step synthesis of size-tunable Ag nanoparticles incorporated in electrospun PVA/cyclodextrin nanofibers. Carbohydr. Polym. 2014, 99, 808–816.
- Csoka, L.; Bozanic, D.K.; Nagy, V.; Dimitrijevic-Brankovic, S.; Luyt, A.S.; Grozdits, G.; Djokovic, V. Viscoelastic properties and antimicrobial activity of cellulose fiber sheets impregnated with Ag nanoparticles. Carbohydr. Polym. 2012, 90, 1139–1146.
- Emam, H.E.; Mowafi, S.; Mashaly, H.M.; Rehan, M. Production of antibacterial colored viscose fibers using in situ prepared spherical Ag nanoparticles. Carbohydr. Polym. 2014, 110, 148–155.
- Ilic, V.; Saponjic, Z.; Vodnik, V.; Potkonjak, B.; Jovancic, P.; Nedeljkovic, J.; Radetic, M. The influence of silver content on antimicrobial activity and color of cotton fabrics functionalized with Ag nanoparticles. Carbohydr. Polym. 2009, 78, 564–569.
- Lavorgna, M.; Attianese, I.; Buonocore, G.G.; Conte, A.; Del Nobile, M.A.; Tescione, F.; Amendola, E. MMT-supported Ag nanoparticles for chitosan nanocomposites: Structural properties and antibacterial activity. Carbohydr. Polym. 2014, 102, 385–392.
- Gondikas, A.P.; Morris, A.; Reinsch, B.C.; Marinakos, S.M.; Lowry, G.V.; Hsu-Kim, H. Cysteine-induced modifications of zero-valent silver nanomaterials: Implications for particle surface chemistry, aggregation, dissolution, and silver speciation. Environ. Sci. Technol. 2012, 46, 7037–7045.
- Qu, F.; Ding, Y.; Lv, X.; Xia, L.; You, J.; Han, W. Emissions of terbium metal-organic frameworks modulated by dispersive/agglomerated gold nanoparticles for the construction of prostate-specific antigen biosensor. Anal. Bioanal. Chem. 2019, 411, 3979–3988.
- Al-Enizi, A.M.; Ahamad, T.; Al-Hajji, A.B.; Ahmed, J.; Chaudhary, A.A.; Alshehri, S.M. Cellulose gum and copper nanoparticles based hydrogel as antimicrobial agents against urinary tract infection (UTI) pathogens. Int. J. Biol. Macromol. 2018, 109, 803–809.
- Guan, G.; Zhang, S.-Y.; Cai, Y.; Liu, S.; Bharathi, M.S.; Low, M.; Yu, Y.; Xie, J.; Zheng, Y.; Zhang, Y.-W.; et al. Convenient purification of gold clusters by co-precipitation for improved sensing of hydrogen peroxide, mercury ions and pesticides. Chem. Commun. 2014, 50, 5703–5705.
- Alavi, M. Modifications of microcrystalline cellulose (MCC), nanofibrillated cellulose (NFC), and nanocrystalline cellulose (NCC) for antimicrobial and wound healing applications. E-Polymers 2019, 19, 103–119.
- Jain, A.S.; Pawar, P.S.; Sarkar, A.; Junnuthula, V.; Dyawanapelly, S. Bionanofactories for green synthesis of silver nanoparticles: Toward antimicrobial applications. Int. J. Mol. Sci. 2021, 22, 11993.
- Tripathi, D.; Modi, A.; Narayan, G.; Rai, S.P. Green and cost effective synthesis of silver nanoparticles from endangered medicinal plant Withania coagulans and their potential biomedical properties. Mater. Sci. Eng. C-Mater. Biol. Appl. 2019, 100, 152–164.
- Lin, F.; Wang, Z.; Shen, Y.; Tang, L.; Zhang, P.; Wang, Y.; Chen, Y.; Huang, B.; Lu, B. Natural skin-inspired versatile cellulose biomimetic hydrogels. J. Mater. Chem. A 2019, 7, 26442–26455.
- Bundjaja, V.; Santoso, S.P.; Angkawijaya, A.E.; Yuliana, M.; Soetaredjo, F.E.; Ismadji, S.; Ayucitra, A.; Gunarto, C.; Ju, Y.-H.; Ho, M.-H. Fabrication of cellulose carbamate hydrogel-dressing with rarasaponin surfactant for enhancing adsorption of silver nanoparticles and antibacterial activity. Mater. Sci. Eng. C-Mater. Biol. Appl. 2021, 118, 111542–111583.
- Liu, Y.; Wang, S.; Wang, Z.; Yao, Q.; Fang, S.; Zhou, X.; Yuan, X.; Xie, J. The in situ synthesis of silver nanoclusters inside a bacterial cellulose hydrogel for antibacterial applications. J. Mater. Chem. B 2020, 8, 4846–4850.
- Liu, R.; Dai, L.; Si, C.; Zeng, Z. Antibacterial and hemostatic hydrogel via nanocomposite from cellulose nanofibers. Carbohydr. Polym. 2018, 195, 63–70.
- Sabbagh, F.; Muhamad, I.I. Acrylamide-based hydrogel drug delivery systems: Release of acyclovir from MgO nanocomposite hydrogel. J. Taiwan Inst. Chem. Eng. 2017, 72, 182–193.
- Dizaj, S.M.; Lotfipour, F.; Barzegar-Jalali, M.; Zarrintan, M.H.; Adibkia, K. Antimicrobial activity of the metals and metal oxide nanoparticles. Mater. Sci. Eng. C-Mater. Biol. Appl. 2014, 44, 278–284.
- Gupta, V.; Singh, S.; Rawat, K.; Bohidar, H.B.; Solanki, P.R. Cytotoxicity and antimicrobial activity of transition metal oxide nanoparticles. Natl. Conf. Nanotechnol. Renew. Energy (NCNRE) 2014, 20, 1650–1653.
- Hrenovic, J.; Milenkovic, J.; Daneu, N.; Kepcija, R.M.; Rajic, N. Antimicrobial activity of metal oxide nanoparticles supported onto natural clinoptilolite. Chemosphere 2012, 88, 1103–1107.
- Azam, A.; Ahmed, A.S.; Oves, M.; Khan, M.S.; Habib, S.S.; Memic, A. Antimicrobial activity of metal oxide nanoparticles against Gram-positive and Gram-negative bacteria: A comparative study. Int. J. Nanomed. 2012, 7, 6003–6009.
- Bhanjana, G.; Kumar, N.; Thakur, R.; Dilbaghi, N.; Kumar, S. Antimicrobial activity of metal & metal oxide nanoparticles interfaced with ligand complexes of 8-hydroxyquinoline and alpha-amino acids. Int. Conf. Adv. Condens. Nano Mater. (ICACNM) 2011, 1393, 1393–1395.
- Applerot, G.; Lipovsky, A.; Dror, R.; Perkas, N.; Nitzan, Y.; Lubart, R.; Gedanken, A. Enhanced antibacterial activity of nanocrystalline ZnO due to increased ROS-mediated cell injury. Adv. Funct. Mater. 2009, 19, 842–852.
- Yadollahi, M.; Gholamali, I.; Namazi, H.; Aghazadeh, M. Synthesis and characterization of antibacterial carboxymethyl cellulose/ZnO nanocomposite hydrogels. Int. J. Biol. Macromol. 2015, 74, 136–141.
- George, D.; Maheswari, P.U.; Sheriffa Begum, K.M.M.; Arthanareeswaran, G. Biomass-derived dialdehyde cellulose cross-linked chitosan-based nanocomposite hydrogel with phytosynthesized zinc oxide nanoparticles for enhanced curcumin delivery and bioactivity. J. Agric. Food Chem. 2019, 67, 10880–10890.
- El Fawal, G.F.; Abu-Serie, M.M.; Hassan, M.A.; Elnouby, M.S. Hydroxyethyl cellulose hydrogel for wound dressing: Fabrication, characterization and in vitro evaluation. Int. J. Biol. Macromol. 2018, 111, 649–659.
- Zhang, H.; Zhu, J.; Hu, Y.; Chen, A.; Zhou, L.; Gao, H.; Liu, Y.; Liu, S. Study on photocatalytic antibacterial and sustained-release properties of cellulose/TiO2/beta-CD composite hydrogel. J. Nanomater. 2019, 2019, 2326042.
- Dharmalingam, K.; Bordoloi, D.; Kunnumakkara, A.B.; Anandalakshmi, R. Preparation and characterization of cellulose-based nanocomposite hydrogel films containing CuO/Cu2O/Cu with antibacterial activity. J. Appl. Polym. Sci. 2020, 137, 49216–49229.
- Gao, W.; Chen, Y.; Zhang, Y.; Zhang, Q.; Zhang, L. Nanoparticle-based local antimicrobial drug delivery. Adv. Drug Deliv. Rev. 2018, 127, 46–57.
- Wang, S.; Gao, Y.; Jin, Q.; Ji, J. Emerging antibacterial nanomedicine for enhanced antibiotic therapy. Biomater. Sci. 2020, 8, 6825–6839.
- Hoque, J.; Bhattacharjee, B.; Prakash, R.G.; Paramanandham, K.; Haldar, J. Dual function injectable hydrogel for controlled release of antibiotic and local antibacterial therapy. Biomacromolecules 2018, 19, 267–278.
- Xiong, M.-H.; Bao, Y.; Yang, X.-Z.; Zhu, Y.-H.; Wang, J. Delivery of antibiotics with polymeric particles. Adv. Drug Deliv. Rev. 2014, 78, 63–76.
- Taccone, F.S.; Bond, O.; Cavicchi, F.Z.; Hites, M. Individualized antibiotic strategies. Curr. Opin. Anesthesiol. 2016, 29, 166–171.
- Felton, T.W.; Hope, W.W.; Roberts, J.A. How severe is antibiotic pharmacokinetic variability in critically ill patients and what can be done about it? Diagn. Microbiol. Infect. Dis. 2014, 79, 441–447.
- Sattari, S.; Tehrani, A.D.; Adeli, M. pH-responsive hybrid hydrogels as antibacterial and drug delivery systems. Polymers 2018, 10, 660.
- Xu, W.; Dong, S.; Han, Y.; Li, S.; Liu, Y. Hydrogels as antibacterial biomaterials. Curr. Pharm. Des. 2018, 24, 843–854.
- Forero-Doria, O.; Polo, E.; Marican, A.; Guzman, L.; Venegas, B.; Vijayakumar, S.; Wehinger, S.; Guerrero, M.; Gallego, J.; Duran-Lara, E.F. Supramolecular hydrogels based on cellulose for sustained release of therapeutic substances with antimicrobial and wound healing properties. Carbohydr. Polym. 2020, 242, 116383–116393.
- Iman, M.; Barati, A.; Safari, S. Characterization, in vitro antibacterial activity, and toxicity for rat of tetracycline in a nanocomposite hydrogel based on PEG and cellulose. Cellulose 2019, 27, 347–356.
- Patwa, R.; Zandraa, O.; Capakova, Z.; Saha, N.; Saha, P. Effect of iron-oxide nanoparticles impregnated bacterial cellulose on overall properties of alginate/casein hydrogels: Potential injectable biomaterial for wound healing applications. Polymers 2020, 12, 2690.
- Sadeghi, S.; Nourmohammadi, J.; Ghaee, A.; Soleimani, N. Carboxymethyl cellulose-human hair keratin hydrogel with controlled clindamycin release as antibacterial wound dressing. Int. J. Biol. Macromol. 2020, 147, 1239–1247.
- Johnson, A.; Kong, F.; Miao, S.; Lin, H.V.; Thomas, S.; Huang, Y.C.; Kong, Z.L. Therapeutic effects of antibiotics loaded cellulose nanofiber and kappa-carrageenan oligosaccharide composite hydrogels for periodontitis treatment. Sci. Rep. 2020, 10, 18037–18059.
- Raza, S.; Matula, K.; Karon, S.; Paczesny, J. Resistance and adaptation of bacteria to non-antibiotic antibacterial agents: Physical stressors, nanoparticles, and bacteriophages. Antibiotics 2021, 10, 435.
- Fatima, F.; Siddiqui, S.; Khan, W.A. Nanoparticles as novel emerging therapeutic antibacterial agents in the antibiotics resistant era. Biol. Trace Elem. Res. 2021, 199, 2552–2564.
- Levard, C.; Hotze, E.M.; Lowry, G.V.; Brown, G.E., Jr. Environmental transformations of silver nanoparticles: Impact on stability and toxicity. Environ. Sci. Technol. 2012, 46, 6900–6914.
- Kai, J.; Zhou, X. Preparation, characterization, and cytotoxicity evaluation of zinc oxide-bacterial cellulose-chitosan hydrogels for antibacterial dressing. Macromol. Chem. Phys. 2020, 221, 2000257–2000268.
- Raho, R.; Nhu, Y.N.; Zhang, N.; Jiang, W.; Sannino, A.; Liu, H.; Pollini, M.; Paladini, F. Photo-assisted green synthesis of silver doped silk fibroin/carboxymethyl cellulose nanocomposite hydrogels for biomedical applications. Mater. Sci. Eng. C-Mater. Biol. Appl. 2020, 107, 110219–110231.
- Ur Rehman, M.S.; Rashid, N.; Ashfaq, M.; Saif, A.; Ahmad, N.; Han, J.-I. Global risk of pharmaceutical contamination from highly populated developing countries. Chemosphere 2015, 138, 1045–1055.
- Cheesman, M.J.; Ilanko, A.; Blonk, B.; Cock, I.E. Developing new sntimicrobial therapies: Are synergistic combinations of plant extracts/compounds with conventional antibiotics the solution? Pharmacogn. Rev. 2017, 11, 57–72.
- Khan, F.; Pham, D.T.N.; Oloketuyi, S.F.; Kim, Y.-M. Antibiotics application strategies to control biofilm formation in pathogenic bacteria. Curr. Pharm. Biotechnol. 2020, 21, 270–286.
- Bajpai, S.K.; Pathak, V.; Soni, B. Minocycline-loaded cellulose nano whiskers/poly(sodium acrylate) composite hydrogel films as wound dressing. Int. J. Biol. Macromol. 2015, 79, 76–85.
- Shukla, S.C.; Singh, A.; Pandey, A.K.; Mishra, A. Review on production and medical applications of epsilon-polylysine. Biochem. Eng. J. 2012, 65, 70–81.
- Alavi, M.; Nokhodchi, A. An overview on antimicrobial and wound healing properties of ZnO nanobiofilms, hydrogels, and bionanocomposites based on cellulose, chitosan, and alginate polymers. Carbohydr. Polym. 2020, 227, 115349–115354.
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632.
- Sangaj, N.S.; Malshe, V.C. Permeability of polymers in protective organic coatings. Prog. Org. Coat. 2004, 50, 28–39.
- Wang, C.; Wang, L.; Zhang, Q.; Cheng, L.; Yue, H.; Xia, X.; Zhou, H. Preparation and characterization of apoacynum venetum cellulose nanofibers reinforced chitosan-based composite hydrogels. Colloids Surf. B-Biointerfaces 2021, 199, 111441–111447.
- Raskin, I.; Ribnicky, D.M.; Komarnytsky, S.; Ilic, N.; Poulev, A.; Borisjuk, N.; Brinker, A.; Moreno, D.A.; Ripoll, C.; Yakoby, N.; et al. Plants and human health in the twenty-first century. Trends Biotechnol. 2002, 20, 522–531.
- Felix, G.; Soto-Robles, C.A.; Nava, E.; Lugo-Medina, E. Principal metabolites in extracts of different plants responsible for antibacterial effects. Chem. Res. Toxicol. 2021, 34, 1970–1983.
- Chen, L.; Pang, Y.; Luo, Y.; Cheng, X.; Lv, B.; Li, C. Separation and purification of plant terpenoids from biotransformation. Eng. Life Sci. 2021, 21, 724–738.
- Zhu, H.-G.; Tang, H.-Q.; Cheng, Y.-Q.; Li, Z.-G.; Tong, L.-T. Electrostatic separation technology for obtaining plant protein concentrates: A review. Trends Food Sci. Technol. 2021, 113, 66–76.
- Mahomoodally, M.F.; Aumeeruddy, M.Z.; Rengasamy, K.R.R.; Roshan, S.; Hammad, S.; Pandohee, J.; Hu, X.; Zengin, G. Ginger and its active compounds in cancer therapy: From folk uses to nano-therapeutic applications. Semin. Cancer Biol. 2021, 69, 140–149.
- Gupta, A.; Briffa, S.M.; Swingler, S.; Gibson, H.; Kannappan, V.; Adamus, G.; Kowalczuk, M.; Martin, C.; Radecka, I. Synthesis of silver nanoparticles using curcumin-cyclodextrins loaded into bacterial cellulose-based hydrogels for wound dressing applications. Biomacromolecules 2020, 21, 1802–1811.
