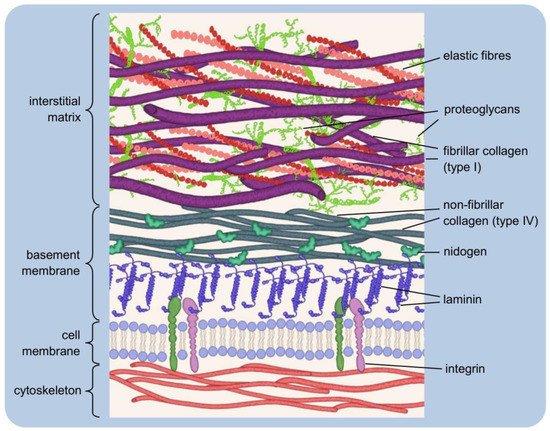
2.1. Host Response to Implantation
The body’s first biological reaction to an implant is forming a layer of water on its surface. This happens in just a few nanoseconds. Water molecules form a mono- or bilayer, and the way they are ordered is strongly dependent on surface properties at the atomic level. Water molecules can dissociate on a highly reactive substrate, resulting in the hydroxylation of the implant surface, i.e., it becomes covered with -OH groups. Water molecules can also be strongly bound but not dissociate. Both of these cases occur as a result of contact with a hydrophilic surface. If the surface is hydrophobic, its interactions with water are much weaker. Therefore, the strength of water-binding determines hydrophobicity or hydrophilicity to the surface. It influences the value of the wetting angle formed between the solid and the plane tangent to the droplet deposited on it. Hydrated ions, such as Cl
−, Na
+, Ca
2+, enter the formed water layer [
403,
404].
Once the aqueous layer covers the material’s surface, proteins from body fluids (extravasated blood/tissue fluid) reach it. In the first stage, mainly smaller proteins with the highest mobility are adsorbed, resulting from faster diffusion of small than large molecules. It is a transient state. A dynamic adsorption–desorption equilibrium is established at the contact surface, as proteins with larger size and a stronger affinity for the implanted material, arriving late, can force the desorption of smaller, weak-bound molecules. This phenomenon is called the Vroman effect. It should be kept in mind that fluids in contact with the implant, such as plasma, contain hundreds of different proteins competing for access to the surface. Therefore, the adsorption–desorption process is much more complex and depends on factors, such as the protein concentration in the fluid. The higher the concentration, the greater the primary surface dominance [
404,
405].
Proteins usually have an asymmetric structure in which domains of different chemical nature can be distinguished. They have a more or less ellipsoidal shape (globular proteins) [
406]. As a result of adsorption, conformational changes of the molecule can occur if it is sufficiently susceptible. It is the effect of binding to the substrate with a privileged side in a given case. As a result, the molecule adopts a certain orientation where part of it invariably contacts the body fluid [
407,
408,
409]. Structurally stable proteins do not readily undergo conformational changes. Their adsorption may occur along the longest axis (“side-on”). Otherwise, this axis is perpendicular to the implant surface (“end-on”) [
407]. The issue is not insignificant in the context of establishing a dynamic adsorption–desorption equilibrium, as the ability to structurally reorient increases the possibility of contact with the substrate [
407,
410].
A major problem with implantation is the foreign body response (FBR), a complex process involving different cell types. Neutrophils are the first to reach the implant site and adhere (via proteins) to the protein-coated surface of the material. Activated neutrophils attempt to degrade the implant by secreting factors, such as proteolytic enzymes or reactive oxygen species. They release chemokines that attract other immune cells, mainly monocytes [
411,
412,
413]. These, in turn, reaching their target, differentiate into macrophages [
414]. The number of macrophages at the implantation site increases due to their progressive proliferation. They replace the initial wave of nucleophiles and release further pro-inflammatory factors. It may lead to implant damage and/or the release of toxic substances into the surrounding tissue environment [
415,
416]. Macrophages may fuse into foreign body giant cells (FBGCs) due to chronic cytokine activity. FGBCs can adhere to the material’s surface for an extended time, leading to collagen deposition and fibrous encapsulation (approximately 3–4 weeks after implantation). As a result, the implant is isolated from the surrounding tissues. It prevents integration and vascularisation and ultimately leads to implant loss [
412,
417]. The fibrous layer is usually thinner on porous than on solid materials [
418,
419]. The presence of mast cells, degranulating upon activation, is also characteristic at the implant site. Among other things, histamine is released from the granules. Histamine dilates blood vessels, improves their permeability and facilitates the arrival of other immune cells. Pro- and anti-inflammatory cytokines and angiogenic or profibrotic factors are also secreted [
420,
421].
Immunosuppressive drugs are used to weaken the body’s immune response and prevent implant rejection. A more recent solution is to incorporate anti-inflammatory agents into the implanted material. They must be released in a controlled manner and at an appropriate rate. An additional requirement is to promote angiogenesis [
422,
423]. For years, biomaterials engineering has been focused on obtaining biologically inert materials, i.e., minimising the interaction with the organism and reducing the immune response. The contemporary trend is the generation of biomimetic materials, i.e., mimicking the natural solutions of the organism and stimulating the desired responses. These include enhancing or inhibiting the normal functioning of immune cells [
424,
425,
426].
2.2. Influence of Material Properties on Cell Adhesion
Cells do not experience direct contact with the implanted material but are only ‘informed’ of its physicochemical properties via proteins deposited on the surface. One of the more important characteristics of the material is the wettability of its surface, which, in the case of an aqueous environment, can be equated with hydrophilicity. It is assumed that the ability of cells to adhere increases on hydrophilic surfaces and decreases on hydrophobic surfaces, even though it is hydrophobic surfaces that are generally considered to be more protein-adsorbent [
427].
The surface protein layer that forms shortly after implantation consists mainly of albumin, fibrinogen, immunoglobulin G, fibronectin, vitronectin et al. The first interactions are usually dominated by albumin due to its relatively small size (66 kDa) and high-concentration in plasma [
411,
428,
429]. It binds much more readily to hydrophobic than hydrophilic surfaces but does not promote cell adhesion. The strong adsorption of albumin reduces the likelihood of being replaced by larger adhesion-promoting proteins, such as fibronectin and vitronectin [
430,
431,
432]. The ability of fibronectin to displace surface-bound albumin is limited on hydrophobic surfaces. As a result of the strong binding of albumin molecules, changes in their secondary structure occur and the degree of denaturation increases [
433]. Proteins tend to denature as the contact time with the material increases, which occurs when albumin adsorbs onto a hydrophobic material. The binding energy of the adsorbed phase then increases, and, as a result, the probability of desorption decreases [
406].
Adsorption occurs more readily if there is a charge difference between the protein molecules and the material surface [
434]. Furthermore, the affinity of the protein for the material may show greater specificity than the distinction between hydrophobicity/hydrophilicity and be based on the recognition of specific functional groups [
429,
433]. Additionally, the cells themselves, depending on the type, show a different preference for the functionality of the surface groups [
435,
436,
437,
438].
Adhesion of cells to the implant surface is made possible by integrins recognising and binding to specific amino acid sequences in the polypeptide chain of the adsorbed protein. It mimics the formation of integrin connections with the ECM proteins under natural conditions. The best known among the pro-adhesive sequences is the tripeptide RGD (arginine-glycine-aspartic acid), present, e.g., in the structure of fibronectin [
439,
440]. One way to modify the material to increase biocompatibility is the coating of tripeptide RGD on its surface in the form of immobilised proteins or short synthetic polypeptide ligands. In addition to RGD, the collagen peptide GFOGER (glycine-phenylalanine-hydroxyproline-glycine-glutamate-arginine) and the laminin-specific sequences IKVAV (isoleucine-lysine-valine-alanine-valine) and YIGSR (tyrosine-isoleucine-glycine-serine-arginine), among others, have been identified [
441,
442,
443,
444,
445].
Functionalisation of the implant surface with peptides containing the RGD sequence has drawbacks. Integrins that recognise RGD may require the presence of other peptides (synergistic effect) to form a bond. The biological activity of short synthetic peptides is less than that of a whole protein. In turn, modification of these peptides (e.g., by chain elongation) can also result in an undesirable change (increase/reduction) in their activity. Another problem is that cells adhere too strongly to the surface, reducing their movement ability [
446,
447,
448].
An interesting conclusion is provided by the study of cell adhesion on materials exhibiting extreme wettability types. Superhydrophobic surfaces are characterised by a water contact angle value higher than 150°, while superhydrophilic surfaces are around 0°. Although the type of cell determines the contact behaviour, only a few show good adhesion to a surface if the material is superhydrophobic. If the surface has highly hydrophilic and hydrophobic regions, cells will usually selectively attach to the superhydrophilic areas [
449].
A significant feature of an implant is the topography of its surface, which, like the chemical composition, influences the interactions with integrins and ultimately stimulates the cellular response [
450]. The shape of the natural matrix at the micro- and nanoscale is understood to be the structure formed by the ECM proteins and the neighbouring cells. For synthetic materials, it is the degree of roughness, the type and size of patterns on the surface. Modifications of these features at the nanoscale affect the activity of the adsorbing proteins by forcing specific changes in their conformation. However, the detailed investigation of such relationships is complicated because the cellular response is always a resultant of the influence of different stimuli. In addition, modifications of topography may be accompanied by changes in surface chemistry [
451,
452,
453,
454,
455].
Techniques to create micropatterns on substrates can be divided into two main types: (1) coating portions of the material with an agent that promotes selective adhesion or (2) applying a layer that blocks adhesion and subsequently removing it without harming the cells embedded around it [
456]. The resulting pattern geometry influences the subsequent formation of cells, e.g., it promotes cell elongation. Furthermore, it supports/inhibits the spreading of cells on the surface. It is related to facilitating/hindering their movement, respectively, depending on the continuity of the pattern [
457]. The size of the contact area between cells can influence their differentiation, i.e., result in different types of daughter cells [
458]. Discontinuities in topography are the cause of local differences in surface free energy. If the cell can detect it, it will modify the contact orientation by reorganising its cytoskeleton. Mechanical signals transmitted to the cell nucleus affect changes at the level of gene transcription and consequently determine cell behaviour. However, the mechanisms underlying the cellular response are still poorly understood [
459,
460].
In addition to patterns characterised by uniformity of shape and size, cell adhesion is influenced by the surface roughness, understood as the overall three-dimensional topography of the substrate, regardless of its regularity. The surfaces of the used materials are rarely smooth at the molecular level, while roughness is not uniformly describable in this case. Cells must be able to recognise a rough surface to react in a certain way, which is dependent on the cell type, as the primary determining factor is the size of the cell. It means that a cell will recognise a surface as smooth if the peak-to-peak distance is greater than the size of the cell [
461,
462,
463].