Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Others
|
Infectious Diseases
A vital factor of viral pathogenesis involves the angiotensin-converting enzyme-2 (ACE2) cellular receptor, which facilitates entry of the virus into susceptible cells. The receptor-binding domain of the spike protein binds to the ACE2 receptor activating membrane fusion of the virus to the host cell. Subsequently, viral RNA is released into the cytoplasm, and the infection is established. The gut microbiota is shaped by our diet; therefore, a healthy gut is important for optimal metabolism, immunology and protection of the host.
- gut microbiota
- SARS-CoV-2
- immunotoxicity
- nutrition
1. Introduction
Since its outbreak in China, the coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has dramatically disseminated throughout the world. This highly pathogenic positive-sense RNA virus consists of structural proteins, namely spike, membrane, nucleocapsid and envelope proteins [1][2]. A vital factor of viral pathogenesis involves the angiotensin-converting enzyme-2 (ACE2) cellular receptor [3], which facilitates entry of the virus into susceptible cells. The receptor-binding domain of the spike protein binds to the ACE2 receptor activating membrane fusion of the virus to the host cell. Subsequently, viral RNA is released into the cytoplasm, and the infection is established [4]. Interestingly, apart from the lungs, the ACE2 receptor is also expressed in the kidney, gastrointestinal tract [5] and the enterocytes of the ileum and colon [6]. COVID-19 mainly targets the respiratory tract; however, it may fatally progress to multiple organ failure [7]. Although effective vaccines are now available, they do not provide 100% protection against COVID-19 infection; therefore, other intervention strategies should be explored to reduce the severity of the disease. Since certain foods demonstrate beneficial immune responses to respiratory viruses, the diet may be proposed to ease the adverse health consequences of COVID-19 [8][9].
The consequence of viral infections is highly dependent on the host’s nutritional status, as the body is exposed to a significant energetic effort to endure the defences [10]. The gut and commensal microbiota can regulate and be regulated by invasive infections, inducing a positive or suppressive result on the host [11]. To overcome the response to pathogens, a healthy gut microbiome is vital for maintaining an optimal immune system and avoiding immune responses that can prove deleterious to the lungs and other organs [12]. Therefore, it may be plausible to consider the gut for a solution to mitigate SARS-CoV-2 infection. It is also evident that the intestinal tract is a COVID-19 infection target as some infected patients present with vomiting and diarrhoea [13], while infected patients recently presented the SARS-CoV-2 RNA in their stool samples [14].
Diet maintains a vital role in human health as it can either affect the gut microbiota by altering physiological responses of the host or by directly attacking the host response [15]. The complex and dynamic mammalian gut microbial community is important for the maturation and development of mucosal and systemic immune responses [15]. The interplay among the microbiota, consumed nutrients and the immune system thus serve as regulators for homeostasis maintenance and protection from invasive pathogens [15]. During the infection process, it is presumed that the enterocytes are infected, thereby compromising the function of intestinal membranes. The intestine acts as a barrier to inhibit microorganisms and its products from leaking into the bloodstream, which is associated with the cytokine storm [16]. Bacteria in the gut produce pathogen-activated molecular patterns (PAMPs), which induce different immune responses via Toll-like receptors (TLRs) [17], depending on the cell, ligand or receptor type [12]. Inflammatory cytokines are released during SARS-CoV-2 injury, leading to a cytokine storm, which initiates an immune dysregulation through T cells and inflammatory monocytes [18]. Cytokines regulate the body’s response to infections and inflammation, and the production of cytokines is impacted by the gut microbial metabolic processes [19]. Modern lifestyles, which include sleep deprivation, daily stress and unbalanced diets, can influence the onset of a chronic low-inflammation, affecting the immune system negatively [20]. The highest COVID-19 morbidity and mortality are in the elderly, especially those with underlying health conditions related to inflammation and other disorders such as cardiovascular disease and diabetes [21]. Additionally, individuals with these underlying health conditions show a less diverse gut microbiome [22], suggesting a link between aging and shifts in gut diversity and pro-inflammatory states. Nutrition is also directly linked to inflammation and subsequently to immune responses. Malnutrition is a global problem that should not be ignored during the COVID-19 pandemic [23][24]. In malnutrition, the consumption of monotonous diets, abundant in highly processed foods, renders inadequate vitamins and minerals to the host, weakening the immune system and enhancing SARS-CoV-2 susceptibility [10].
2. Regulation of the Gut Microbiota
Viral infections have mostly been documented in terms of the virus, the host cell and the host immune system. However, over the past decade, viral infections have been affiliated with the term “microbiota revolution”, which links several pathological manifestations to the gut microbiota and its alterations [25]. The microbiota is a complex group of microorganisms that colonize the mucosal surfaces and are responsible for nutrient absorption and waste secretion [25]. The human gut microbiota contains 1014 resident microorganisms, including fungi, viruses, bacteria and archaea [26]. These microorganisms perform a vital role in health and disease attributing to its metabolic and immunomodulatory activity and protection against pathogens [27]. Commensal bacteria are particularly important for shaping the host immune system and triggering its responses [28][29]. The gut controls the formation and action of the adaptive and innate immune system by tuning immune cells for inflammatory responses and maintaining immune homeostasis [30]. This affects the host’s susceptibility to various diseases; therefore, in SARS-CoV-2 infection, a healthy gut microbiota is crucial for sustaining an optimum immune system which averts uncontrolled inflammatory responses [30]. Modifications of the gut microbiota are characterized by multiple factors, with the main cause being viral infections [25]. Additionally, the gut microbiota also affects pulmonary health via a crosstalk of the lungs and gut microbiota, known as the gut–lung axis [31]. This gut–lung axis is bidirectional; therefore, when microbial endotoxins affect the lungs via the blood causing inflammation, and the gut microbiota may also be affected [32]. The hosts gut microbiota enables the digestion of various dietary products. Dysbiosis of the gut microbiota stimulates mucosal innate immune responses and enhances the permeability of the intestine. This leads to the transfer of pathogenic organisms, allowing detrimental metabolites to access the intestinal epithelium and promoting disease severity [33]. Additionally, a link between ACE2 and the gut microbiota has also been documented. In a mouse model, ACE2 deficiency impaired tryptophan homeostasis, which altered the gut microbiome and inflammatory response [34]. In intestinal epithelial cells, the ACE2 receptor may also control nutrient uptake by attaching to amino acid transporters, suggesting that SARS-CoV-2 may compete against protein nutrients and disrupt absorption via the ACE2 receptor [35][36]. This brings about a possible link between SARS-CoV-2 infections and how the gut microbiota may impact infection severity (Figure 1).
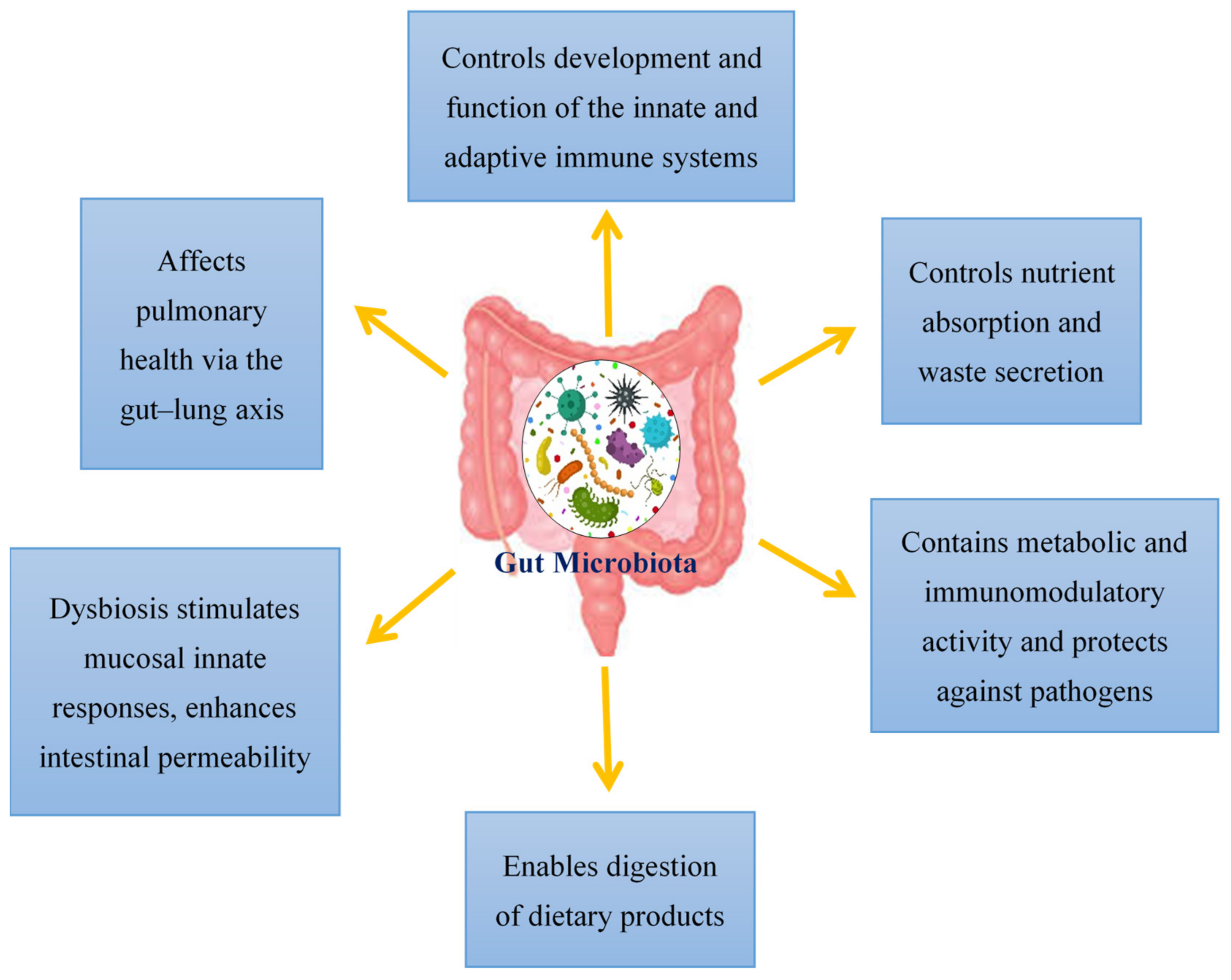
Figure 1. An overview of the functions of the gut microbiota in the host.
3. The Role of ACE2 in the Gut
The type I membrane-anchored glycoprotein, ACE2, encloses 805 amino acids and contains an N-terminal peptidase domain along with a C-terminal collectrin-like domain [37]. ACE2 is a negative regulator of the Renin–Angiotensin System (RAS), thus providing relief from the harmful effects facilitated by angiotensin (Ang) II signalling via the Ang II receptor type I (AT1R) [38]. ACE2 also demonstrates RAS-independent roles which promote intestinal dysbiosis through loss of ACE2 expression or function [39]. This supports the gastrointestinal symptoms experienced by COVID-19 patients [40]. The gut microbiota also controls ACE2 expressions and therefore plays a role in COVID-19 severity and contagion [41].
Activity of intracrine gut ACE2 encompasses modulation of electrolyte homeostasis, gastrointestinal epithelial fluid, gastrointestinal mucosal inflammation, smooth muscle control and gut-specific fibrosis [42]. In the gut, ACE2 shares a site with B0AT1 and functions as a membrane trafficking chaperone of B0AT1, which controls the sodium-dependent uptake of tryptophan and glutamine (neutral amino acids) into intestinal cells [41]. Animal ACE2-knockout studies displayed altered gut microbial composition, decreased tryptophan serum levels and reduced small intestinal antimicrobial peptide (AMPs) expression [34] in addition to reduced AMP results in dysbiosis, enhanced pathogen levels and impaired gut microbiota [43]. It is proposed that AMP expression and composition of the gut microbiota are regulated by mTOR activation via the tryptophan-nicotinamide pathway and/or nutrient sensing (Figure 2) [34][39].
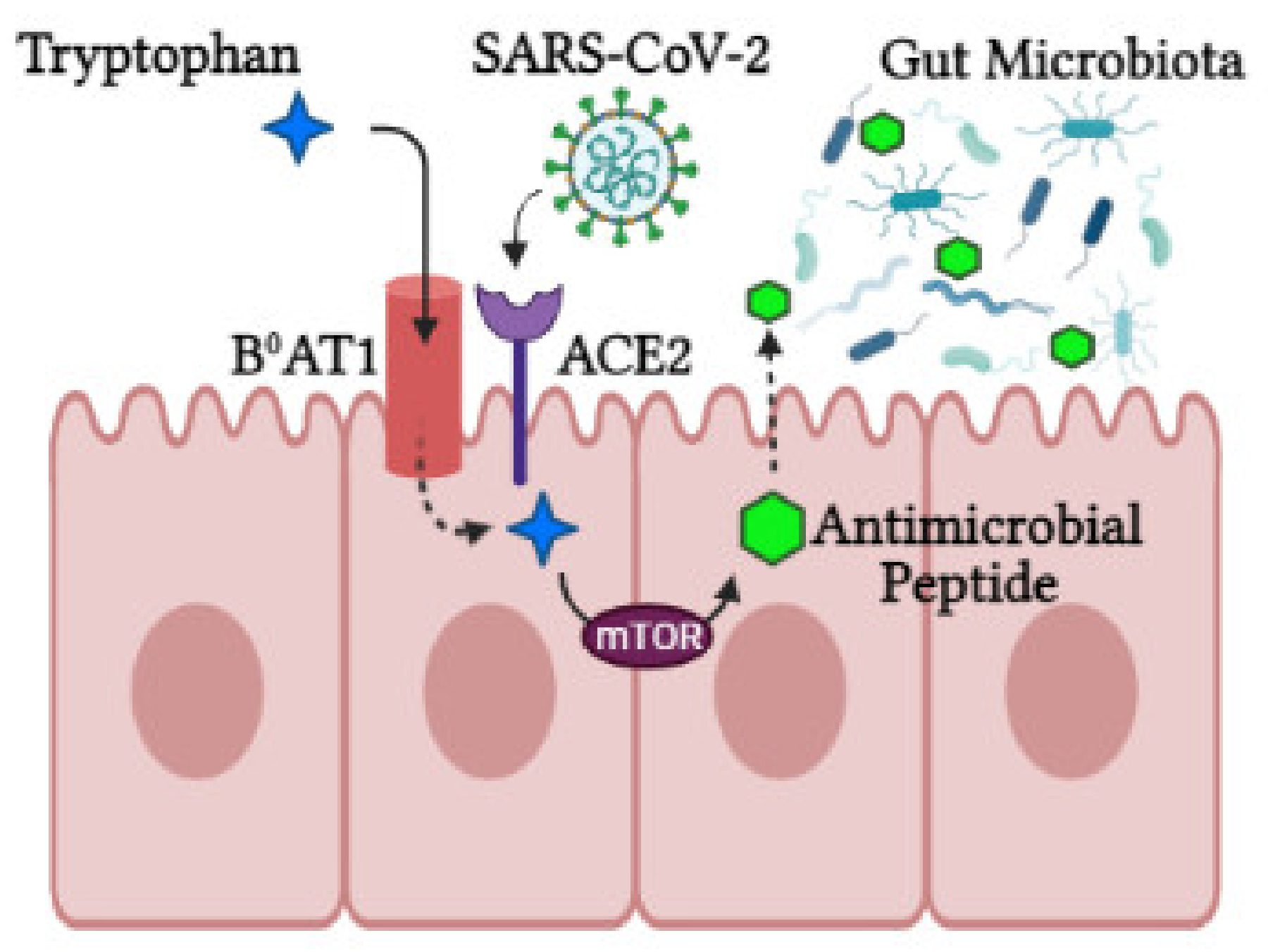
Figure 2. The role of ACE2 in the intestine. ACE2 is required for B0AT1 amino acid transporter that mediates tryptophan uptake. Tryptophan triggers antimicrobial peptide secretion via the mTOR pathway, which can alter the gut microbiota composition. Upon SARS-CoV-2 infection, ACE2 is downregulated, leading to aberrant absorption of tryptophan and antimicrobial peptides. This subsequently alters the gut microbiota, conferring susceptibility to inflammation (created with BioRender.com, accessed on 25 October 2021).
During SARS-CoV-2 infection, luminal ACE2 levels are downregulated, impacting gut permeability, nutrient transport and local and systemic inflammation. In the luminal surface of enterocytes, ACE2 deficiency enhances Ang II and reduces Ang1-7. This Ang1-7 decrease in turn activates AT1R and increases gut permeability linked to leaky gut syndrome [39], which may facilitate a cytokine storm [44]. In addition, ACE2 deficiency downregulates ACE2-B0AT1 complexes, hindering neutral amino acid uptake, which are critical for T-cell function, Toll-like receptor signaling and NF-kβ activation and inflammation [44]. Tryptophan also triggers incretins, which regulate glucose homeostasis and promotes hypoglycemia. Furthermore, loss of ACE2-Mas receptor binding in the gut halts glucose transport mediated by SGLT1 and GLUT luminal glucose transporters [41]. In enterocytes’ luminal surfaces, ACE2 deficiency also involves digestive enzyme degradation to produce free amino acids [41].
This entry is adapted from the peer-reviewed paper 10.3390/ijms23052654
References
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.-H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280.
- Walls, A.C.; Park, Y.J.; Tortorici, M.A.; Wall, A.; McGuire, A.T.; Veesler, D. Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein. Cell 2020, 181, 281–292.
- Zhang, H.; Penninger, J.M.; Li, Y.; Zhong, N.; Slutsky, A.S. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: Molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020, 46, 586–590.
- Ni, W.; Yang, X.; Yang, D.; Bao, J.; Li, R.; Xiao, Y.; Hou, C.; Wang, H.; Liu, J.; Yang, D.; et al. Role of angiotensin-converting enzyme 2 (ACE2) in COVID-19. Crit. Care 2020, 24, 422.
- Leung, W.K.; To, K.F.; Chan, P.K.; Chan, H.L.; Wu, A.K.; Lee, N.; Yuen, K.Y.; Sung, J.J. Enteric involvement of severe acute respiratory syndrome-associated coronavirus infection. Gastroenterology 2003, 125, 1011–1017.
- Zhang, H.; Kang, Z.; Gong, H.; Xu, D.; Wang, J.; Li, Z.; Li, Z.; Cui, X.; Xiao, J.; Zhan, J.; et al. Digestive system is a potential route of COVID-19: An analysis of single-cell coexpression pattern of key proteins in viral entry process. Gut 2020, 69, 1010–1018.
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506.
- Cena, H.; Chieppa, M. Coronavirus Disease (COVID-19–SARS-CoV-2) and Nutrition: Is Infection in Italy Suggesting a Connection? Front. Immunol. 2020, 11, 944.
- Iddir, M.; Brito, A.; Dingeo, G.; Fernandez Del Campo, S.S.; Samouda, H.; La Frano, M.R.; Bohn, T. Strengthening the Immune System and Reducing Inflammation and Oxidative Stress through Diet and Nutrition: Considerations during the COVID-19 Crisis. Nutrients 2020, 12, 1562.
- Briguglio, M.; Pregliasco, F.E.; Lombardi, G.; Perazzo, P.; Banfi, G. The Malnutritional Status of the Host as a Virulence Factor for New Coronavirus SARS-CoV-2. Front. Med. 2020, 7, 146.
- Li, N.; Ma, W.-T.; Pang, M.; Fan, Q.-L.; Hua, J.-L. The Commensal Microbiota and Viral Infection: A Comprehensive Review. Front. Immunol. 2019, 10, 1551.
- Dhar, D.; Mohanty, A. Gut microbiota and Covid-19- possible link and implications. Virus Res. 2020, 285, 198018.
- Lin, L.; Jiang, X.; Zhang, Z.; Huang, S.; Zhang, Z.; Fang, Z.; Gu, Z.; Gao, L.; Shi, H.; Mai, L.; et al. Gastrointestinal symptoms of 95 cases with SARS-CoV-2 infection. Gut 2020, 69, 997–1001.
- Wu, Y.; Guo, C.; Tang, L.; Hong, Z.; Zhou, J.; Dong, X.; Yin, H.; Xiao, Q.; Tang, Y.; Qu, X.; et al. Prolonged presence of SARS-CoV-2 viral RNA in faecal samples. Lancet Gastroenterol. Hepatol. 2020, 5, 434–435.
- Statovci, D.; Aguilera, M.; MacSharry, J.; Melgar, S. The Impact of Western Diet and Nutrients on the Microbiota and Immune Response at Mucosal Interfaces. Front. Immunol. 2017, 8, 838.
- Groschwitz, K.R.; Hogan, S.P. Intestinal barrier function: Molecular regulation and disease pathogenesis. J. Allergy Clin. Immunol. 2009, 124, 3–20.
- Beutler, B. Inferences, questions and possibilities in Toll-like receptor signalling. Nature 2004, 430, 257–263.
- Zhou, Y.; Fu, B.; Zheng, X.; Wang, D.; Zhao, C.; Qi, Y.; Sun, R.; Tian, Z.; Xu, X.; Wei, H. Pathogenic T cells and inflammatory monocytes incite inflammatory storm in severe COVID-19 patients. Natl. Sci. Rev. 2020, 7, 998–1002.
- Kalantar-Zadeh, K.; Ward, S.A.; Kalantar-Zadeh, K.; El-Omar, E.M. Considering the Effects of Microbiome and Diet on SARS-CoV-2 Infection: Nanotechnology Roles. ACS Nano 2020, 14, 5179–5182.
- Haspel, J.A.; Anafi, R.; Brown, M.K.; Cermakian, N.; Depner, C.; Desplats, P.; Gelman, A.E.; Haack, M.; Jelic, S.; Kim, B.S.; et al. Perfect timing: Circadian rhythms, sleep, and immunity—An NIH workshop summary. JCI Insight 2020, 5, e131487.
- Gao, Q.; Hu, Y.; Dai, Z.; Xiao, F.; Wang, J.; Wu, J. The epidemiological characteristics of 2019 novel coronavirus diseases (COVID-19) in Jingmen, Hubei, China. Medicine 2020, 99, e20605.
- Karst, S.M. The influence of commensal bacteria on infection with enteric viruses. Nat. Rev. Microbiol. 2016, 14, 197–204.
- Calder, P.C.; Carr, A.C.; Gombart, A.F.; Eggersdorfer, M. Optimal Nutritional Status for a Well-Functioning Immune System Is an Important Factor to Protect against Viral Infections. Nutrients 2020, 12, 1181.
- Pinheiro, T.A.; Barcala-Jorge, A.S.; Andrade, J.M.O.; Pinheiro, T.A.; Ferreira, E.C.N.; Crespo, T.S.; Batista-Jorge, G.C.; Vieira, C.A.; Lelis, D.F.; Paraíso, A.F.; et al. Obesity and malnutrition similarly alter the renin-angiotensin system and inflammation in mice and human adipose. J. Nutr. Biochem. 2017, 48, 74–82.
- Infusino, F.; Marazzato, M.; Mancone, M.; Fedele, F.; Mastroianni, C.M.; Severino, P.; Ceccarelli, G.; Santinelli, L.; Cavarretta, E.; Marullo, A.G. Diet Supplementation, Probiotics, and Nutraceuticals in SARS-CoV-2 Infection: A Scoping Review. Nutrients 2020, 12, 1718.
- Gill, S.R.; Pop, M.; DeBoy, R.T.; Eckburg, P.B.; Turnbaugh, P.J.; Samuel, B.S.; Gordon, J.I.; Relman, D.A.; Fraser-Liggett, C.M.; Nelson, K.E. Metagenomic Analysis of the Human Distal Gut Microbiome. Science 2006, 312, 1355.
- Lloyd-Price, J.; Abu-Ali, G.; Huttenhower, C. The healthy human microbiome. Genome Med. 2016, 8, 51.
- Hooper, L.V.; Gordon, J.I. Commensal host-bacterial relationships in the gut. Science 2001, 292, 1115–1118.
- Chervonsky, A. Innate receptors and microbes in induction of autoimmunity. Curr. Opin. Immunol. 2009, 21, 641–647.
- He, L.-H.; Ren, L.-F.; Li, J.-F.; Wu, Y.-N.; Li, X.; Zhang, L. Intestinal Flora as a Potential Strategy to Fight SARS-CoV-2 Infection. Front. Microbiol. 2020, 11, 1388.
- Keely, S.; Talley, N.J.; Hansbro, P.M. Pulmonary-intestinal cross-talk in mucosal inflammatory disease. Mucosal Immunol. 2012, 5, 7–18.
- Dumas, A.; Bernard, L.; Poquet, Y.; Lugo-Villarino, G.; Neyrolles, O. The role of the lung microbiota and the gut–lung axis in respiratory infectious diseases. Cell. Microbiol. 2018, 20, e12966.
- Collins, S.M. A role for the gut microbiota in IBS. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 497–505.
- Hashimoto, T.; Perlot, T.; Rehman, A.; Trichereau, J.; Ishiguro, H.; Paolino, M.; Sigl, V.; Hanada, T.; Hanada, R.; Lipinski, S.; et al. ACE2 links amino acid malnutrition to microbial ecology and intestinal inflammation. Nature 2012, 487, 477–481.
- Vuille-dit-Bille, R.N.; Camargo, S.M.; Emmenegger, L.; Sasse, T.; Kummer, E.; Jando, J.; Hamie, Q.M.; Meier, C.F.; Hunziker, S.; Forras-Kaufmann, Z.; et al. Human intestine luminal ACE2 and amino acid transporter expression increased by ACE-inhibitors. Amino Acids 2015, 47, 693–705.
- Javed, K.; Bröer, S. Mice Lacking the Intestinal and Renal Neutral Amino Acid Transporter SLC6A19 Demonstrate the Relationship between Dietary Protein Intake and Amino Acid Malabsorption. Nutrients 2019, 11, 2024.
- Yan, R.; Zhang, Y.; Li, Y.; Xia, L.; Guo, Y.; Zhou, Q. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science 2020, 367, 1444–1448.
- Kuba, K.; Imai, Y.; Rao, S.; Gao, H.; Guo, F.; Guan, B.; Huan, Y.; Yang, P.; Zhang, Y.; Deng, W. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus–induced lung injury. Nat. Med. 2005, 11, 875–879.
- Perlot, T.; Penninger, J.M. ACE2–From the renin–angiotensin system to gut microbiota and malnutrition. Microbes Infect. 2013, 15, 866–873.
- Kotfis, K.; Skonieczna-Żydecka, K. COVID-19: Gastrointestinal symptoms and potential sources of 2019-nCoV transmission. Anaesthesiol. Intensive Ther. 2020, 40157, 2020.
- Sajdel-Sulkowska, E.M. A Dual-Route Perspective of SARS-CoV-2 Infection: Lung- vs. Gut-specific Effects of ACE-2 Deficiency. Front. Pharmacol. 2021, 12, 684610.
- Garg, M.; Angus, P.W.; Burrell, L.M.; Herath, C.; Gibson, P.R.; Lubel, J.S. The pathophysiological roles of the renin–angiotensin system in the gastrointestinal tract. Aliment. Pharmacol. Ther. 2012, 35, 414–428.
- Yang, T.; Santisteban, M.M.; Rodriguez, V.; Li, E.; Ahmari, N.; Carvajal, J.M.; Zadeh, M.; Gong, M.; Qi, Y.; Zubcevic, J.; et al. Gut dysbiosis is linked to hypertension. Hypertension 2015, 65, 1331–1340.
- Ren, W.; Rajendran, R.; Zhao, Y.; Tan, B.; Wu, G.; Bazer, F.W.; Zhu, G.; Peng, Y.; Huang, X.; Deng, J. Amino acids as mediators of metabolic cross talk between host and pathogen. Front. Immunol. 2018, 9, 319.
This entry is offline, you can click here to edit this entry!
