Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Glycolysis and ER stress have been considered important drivers of pulmonary fibrosis. However, it is not clear whether glycolysis and ER stress are interconnected and if those interconnections regulate the development of pulmonary fibrosis.
- oxamate
- glycolysis
- ER stress
1. Introduction
Silicosis is an occupational pneumoconiosis caused by the inhalation of free crystalline silicon dioxide (SiO2) or silica dust [1]. When macrophages phagocytize silica particles, they must quickly adapt their metabolism to provide sufficient energy to maintain their immunomodulatory functions, including phagocytosis and the production of inflammatory cytokines and chemokines [2]. This metabolic switch from oxidative phosphorylation to glycolytic metabolism provides an energy source for sustaining inflammatory damage [3]. We previously demonstrated that the levels of key glycolytic enzymes, including hexokinase2 (HK2), pyruvate kinase M2 (PKM2), lactate dehydrogenase A (LDHA), and the lactate concentration were enhanced in silica-induced alveolar macrophage and silicotic models, suggesting that the metabolic switch to glycolysis is an important driving force for the development of silicosis fibrosis [4].
Endoplasmic reticulum (ER) stress and the unfolded protein response (UPR) have been linked to lung fibrosis through the regulation of alveolar epithelial cell (AEC) apoptosis, epithelial–mesenchymal transition (EMT), fibroblast proliferation, myofibroblast differentiation, and M2 macrophage polarization [5,6]. Our previous studies also found that ER stress was abnormally activated in silica-induced A549 cells [7] and MLE-12 cells [8]. Other studies have shown that ER stress and the UPR were critical to the function and phenotypic transformation of macrophages [9]. However, whether silica exposure increases glycolysis and ER stress in macrophages remains unclear.
Lactate, the end product of glycolysis, was once considered a metabolic waste product [10], but several studies have shown that lactate has multiple important biological functions [11,12]. One study showed that the pulmonary release of lactate was directly proportional to the severity of lung injury [13]. In addition, it was recently shown that lactate induced the production of reactive oxygen species (ROS) and promoted the expression of UPR genes [14]. Our previous studies also found that silica induced an increase in extracellular lactate levels in macrophages [4]. However, it is not clear whether lactate can regulate glycolysis and ER stress in NR8383 macrophages. In addition to lactate production, we also found that the expression of LDHA was increased in silica-induced macrophages, and small interfering RNA (siRNA)-Ldha inhibited the activation of these macrophages, giving them an anti-inflammatory role [4]. However, whether pharmacological inhibition of LDHA plays an anti-fibrotic role by regulating macrophage function remains unclear.
Oxamate, an amide isostere of pyruvate, is a competitive inhibitor of LDHA [15]. Studies have shown that oxamate inhibits the proliferation of nasopharyngeal carcinoma [16], non-small cell lung cancer [17], and gastric cancer cells [18] and decreases their viability. Therefore, it has been suggested that oxamate may be a promising anticancer agent. However, it is not clear whether oxamate has a therapeutic effect on silicosis fibrosis.
2. Silica Increased ER Stress in Macrophages
In our preliminary study, we found that SiO2 induced ER stress in A549 cells [7] and MLE-12 cells [8]. As a follow-up, this study was aimed at further investigating whether ER stress was also manifested in silica-induced macrophages. As shown in Figure 1A, the fluorescence intensity of phospho-PKR-like ER kinase (p-PERK) was significantly enhanced in a dose-dependent manner by supplementation of the macrophages with 100, 200, and 250 µg/mL SiO2. Furthermore, exposure of the macrophages to SiO2 induced an increase in the expression of phospho-inositol-requiring enzyme 1α (p-IRE-1α) (Figure 1B). We also found that SiO2 significantly increased intracellular ROS production (Figure 1C). Western blotting analysis indicated that the expressions of p-PERK, p-IRE-1α, and phospho-eukaryotic initiation factor 2 alpha (p-eIF-2α) were upregulated in a dose-dependent manner by supplementation of the macrophages with 100, 200, and 250 µg/mL SiO2 (Figure 1D). These results suggested that silica induced ER stress in macrophages.
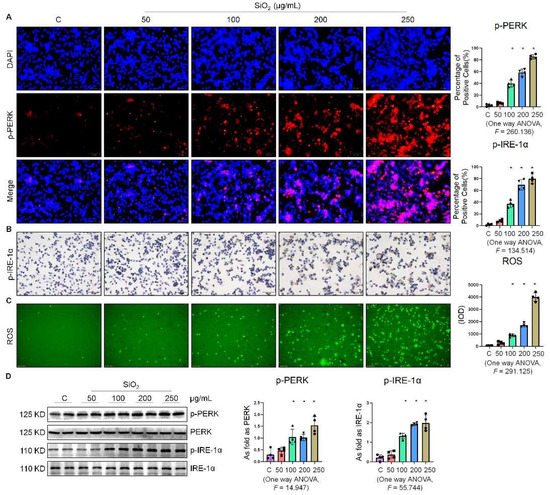
Figure 1. Silica increased ER stress in macrophages. (A) Expression of p-PERK in NR8383 cells treated with SiO2 at different doses observed by immunofluorescence (IF) staining (scale bar = 50 µm). (B) Expression of p-IRE-1α in NR8383 cells treated with SiO2 at different doses observed by immunohistochemistry (IHC) staining (scale bar = 50 µm). (C) Intracellular ROS production in NR8383 cells treated with SiO2 at different doses observed by 2,7-dichlorodihydrofluorescein diacetate (DCFH-DA) staining (scale bar = 100 µm). (D) Protein expression of p-PERK and p-IRE-1α in NR8383 cells treated with SiO2 at different doses measured by Western blotting. * Compared with control group, p < 0.05. Data are presented as the mean ± SD, n = 4 per group.
3. Oxamate Attenuated the Enhancement of Glycolysis and ER Stress in Silica-Induced Macrophages
Since glycolytic reprogramming and ER stress are critical for the functional and phenotypic transformation of macrophages, we further investigated whether the targeted inhibition of LDHA had an effect on the regulation of glycolysis and ER stress. Oxamate alleviated the silica-induced increase in the expression of p-PERK and p-IRE-1α as well as the increase in ROS production (Figure 2A–C). Similarly, oxamate treatment reduced the extent of ER stress by inhibiting the silica-induced upregulation of p-PERK and p-IRE-1α, but not by inhibiting p-eIF-2α (Figure 2D). To investigate the effect of oxamate on metabolic changes in macrophages, we assessed the changes in the extracellular acidification rate (ECAR), lactate concentration, and the mitochondrial oxygen consumption rate (OCR) after treatment of the silicotic macrophages with oxamate. After exposure of the macrophages to silica, we detected increases in both the ECAR and lactate concentration but a decrease in the OCR, indicating the transition from oxidative phosphorylation to aerobic glycolysis. However, these effects were reversed upon treatment of the silica-exposed macrophages with oxamate (Figure 3).
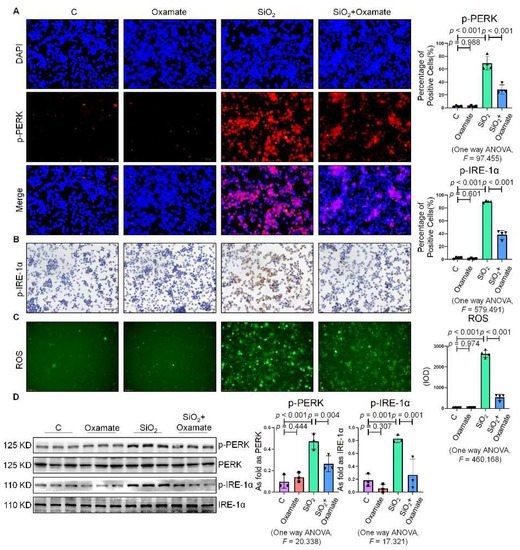
Figure 2. Oxamate attenuated the enhancement of ER stress in silica-induced macrophages. (A) Expression of p-PERK in NR8383 cells observed by IF staining (scale bar = 50 µm). (B) Expression of p-IRE-1α in NR8383 cells observed by IHC staining (scale bar = 50 µm). (C) Intracellular ROS production in NR8383 cells observed by DCFH-DA staining (scale bar = 100 µm). (D) Protein expression of p-PERK and p-IRE-1α in NR8383 cells treated with oxamate, SiO2, and SiO2 plus oxamate measured by Western blotting. Data are presented as the mean ± SD, n = 3 per group.
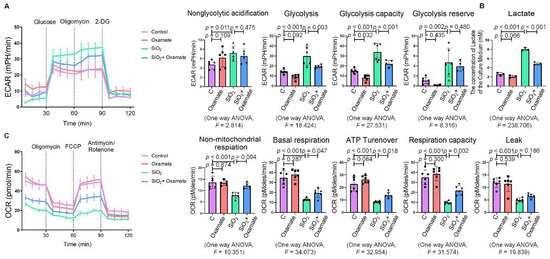
Figure 3. Oxamate attenuated the enhancement of glycolysis in silica-induced macrophages. (A) Glycolysis flux was examined by measuring the ECAR using the Seahorse analyzer. Data are presented as the mean ± SD, n = 6 per group. (B) The lactate concentration in the culture medium was detected using a lactate assay kit. Data are presented as the mean ± SD, n = 3 per group. (C) The OCR values were measured using the Seahorse analyzer. Data are presented as the mean ± SD, n = 6 per group.
4. Lactate Promoted ER Stress in Macrophages
To determine the effect of lactate on the expression of ER stress-related factors in NR8383 cells, we stimulated NR8383 cells with different concentrations of lactate. As shown in Figure 4A,B, the expression of p-PERK and p-IRE-1α gradually increased with the increase in the lactate concentration. It was previously reported that exogenous lactate stimulation resulted in an increase in intercellular ROS production in skin fibroblasts [19]. Our study corroborated this finding, as lactate was also found to stimulate the production of ROS in NR8383 cells (Figure 4C). Supplementation of exogenous lactate triggered an increase in the expression of p-PERK, p-IRE-1α, and p-eIF-2α in NR8383 cells in a dose-dependent manner (Figure 4D). Taken together, these results indicated that lactate played a key role in the activation of ER stress in macrophages.
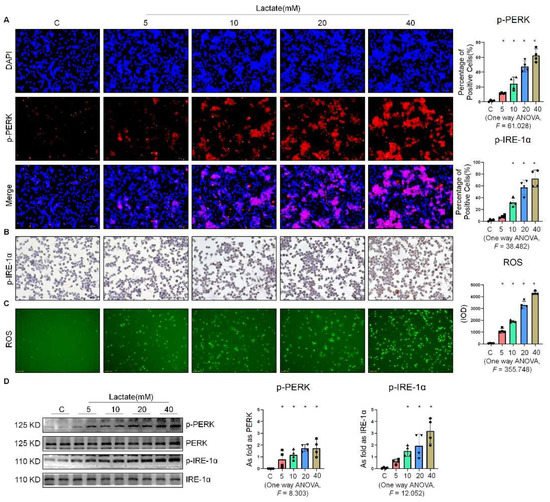
Figure 4. Lactate promoted ER stress in macrophages. (A) Expression of p-PERK in NR8383 cells treated with lactate at different doses observed by IF staining (scale bar = 50 µm). (B) Expression of p-IRE-1α in NR8383 cells treated with lactate at different doses observed by IHC staining (scale bar = 50 µm). (C) Intracellular ROS production in NR8383 cells treated with lactate at different doses observed by DCFH-DA staining (scale bar = 100 µm). (D) Levels of p-PERK and p-IRE-1α in NR8383 cells treated with lactate at different doses measured by Western blotting. * Compared with control group, p < 0.05. All data are presented as the mean ± SD, n = 4 per group.
5. Oxamate Attenuated the Lactate-Induced Enhancement of Glycolysis and ER Stress in Macrophages
Next, we used oxamate to inhibit LDH and prevent the conversion of pyruvate to lactate, reducing the production of lactate. We found that treatment with oxamate inhibited the enhanced expression of p-PERK and p-IRE-1α and ROS production induced by exogenous lactate (Figure 5A–C). In addition, treatment of the silica-exposed macrophages with oxamate inhibited the activation of ER stress by targeting p-PERK and p-IRE-1α rather than p-eIF-2α (Figure 5D). Next, we sought to verify whether oxamate regulated lactate-induced metabolic changes. Treatment of the macrophages with oxamate resulted in lower levels of ECAR, a reduced production of lactate, and higher levels of OCR due to the inhibition of LDHA (Figure 6). These findings indicated that oxamate alleviated the lactate-induced increase in glycolytic metabolism and ER stress by inhibiting LDHA.
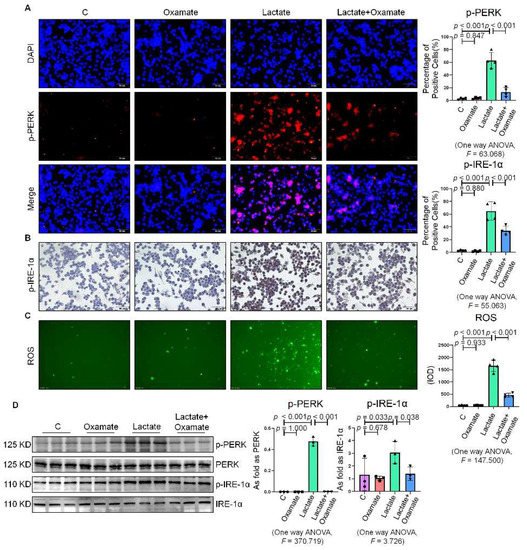
Figure 5. Oxamate attenuated the lactate-induced enhancement of ER stress in macrophages. (A) Expression of p-PERK in NR8383 cells observed by IF staining (scale bar = 50 µm). (B) Expression of p-IRE-1α in NR8383 cells observed by IHC staining (scale bar = 50 µm). (C) Intracellular ROS production in NR8383 cells by using DCFH-DA staining (scale bar = 100 µm). (D) Protein expression of p-PERK and p-IRE-1α in NR8383 cells treated with oxamate, lactate, and lactate plus oxamate measured by Western blotting. Data are presented as the mean ± SD, n = 3 per group.
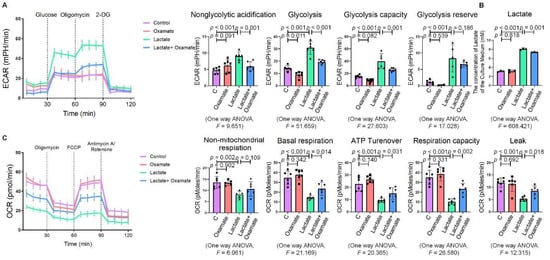
Figure 6. Oxamate attenuated the lactate-induced enhancement of glycolysis in macrophages. (A) Glycolysis flux was examined by measuring the ECAR using the Seahorse analyzer. Data are presented as the mean ± SD, n = 6 per group. (B) The lactate content in the culture medium was detected using a lactate assay kit. Data are presented as the mean ± SD, n =3 per group. (C) The OCR values were measured using the Seahorse analyzer. Data are presented as the mean ± SD, n = 6 per group.
6. Oxamate Reduced Glycolysis and ER Stress in Silicotic Mice
Since we observed that oxamate inhibited glycolysis and ameliorated ER stress in macrophages, we considered whether oxamate could mitigate fibrotic responses to silica in vivo. To test this hypothesis, we administered different doses of oxamate to mice exposed to silica. Treatment of the silicotic mice with 100 mg/kg or 500 mg/kg oxamate per day for 4 weeks led to a significant reduction in the expression of LDHA and attenuation of fibrotic remodeling in the silica-exposed lungs of the mice, as assessed by HE. and Sirius Red histological staining of the lung tissue (Figure 7A–C). Consistent with our in vitro observations, treatment of the mice exposed to silica with 100 mg/kg or 500 mg/kg oxamate led to the reduced expression of p-PERK and p-IRE-1α but had no effect on p-eIF-2α; in addition, the effects of the 500 mg/kg oxamate dosage on the expression of the two proteins were more significant than the 100 mg/kg dosage (Figure 7D–F). Taken together, these data supported that oxamate played an anti-fibrosis role in vivo by inhibiting glycolysis and ER stress in macrophages.
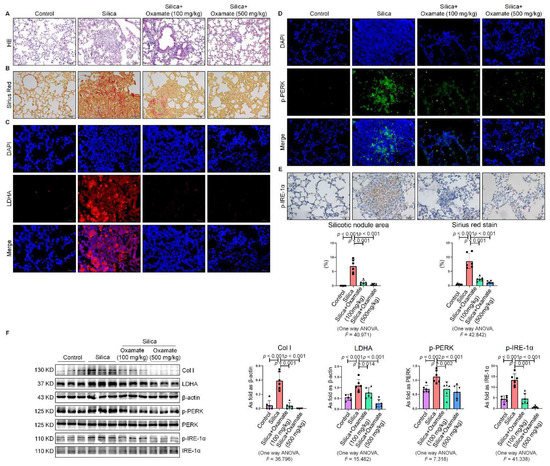
Figure 7. Oxamate reduced glycolysis and ER stress in silicotic mice. (A) HE. staining of lung tissue in mice exposed to silica (scale bar = 100 µm). (B) Sirius red staining of lung tissue in mice exposed to silica (scale bar = 50 µm). (C) Expression of LDHA in mice exposed to silica measured by IF staining (scale bar = 50 µm). (D) Expression of p-PERK in silicotic mice measured by IF staining (scale bar) = 50 µm. (E) Positive expression of p-IRE-1α in silicotic mice observed by IHC staining (scale bar = 50 µm). (F) Expression levels of collagen type I (Col I), LDHA, p-PERK, and p-IRE-1α in mice lungs measured by Western blotting. Data are presented as the mean ± SD, n = 6 per group.
This entry is adapted from the peer-reviewed paper 10.3390/ijms23063013
This entry is offline, you can click here to edit this entry!
