Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Degenerative disc disease (DDD) is one of the most common findings in CLBP patients. DDD is an inflammatory–catabolic process triggered by a long list of genetic, mechanical, and environmental factors that ultimately leads to the resorption of the IVD.
- mesenchymal stem cell
- stem cell therapy
- Modic change
- intervertebral disc
- degenerative disc disease
1. Introduction
A fundamental challenge for improving the lives of chronic low back pain (CLBP) patients is the lack of effective targeted treatments. The development of novel targeted therapies for CLBP patients is hampered in part by the heterogeneity of the CLBP population. Pain may arise from several anatomical structures, including the intervertebral disc (IVD), the endplate, the vertebral body, the facet joints, the spinal ligaments, and the muscles. Central pain sensitization and psychosocial factors can further complicate the diagnosis.
Degenerative disc disease (DDD) is one of the most common findings in CLBP patients. DDD is an inflammatory–catabolic process triggered by a long list of genetic, mechanical, and environmental factors that ultimately leads to the resorption of the IVD. Anti-inflammatory and regenerative approaches have been attempted to treat degenerated discs. In the past 15 years, many cell therapy approaches for DDD have been developed, several of which have reached phase I and II clinical trials, and a few phase III trials [1].
Patient stratification is critical for showing a clinically meaningful treatment effect. However, the high prevalence of disc degeneration (DD) in the heterogeneous CLBP population [2] and the high percentage of asymptomatic individuals with DD (31.5–37.5%) [3] limit the sensitivity and specificity of DD for CLBP and hence impose a major challenge to stratify patients for a potential therapy.
Modic changes (MC) are vertebral bone marrow lesions that are almost exclusively present at levels with DD. MC are frequently observed in CLBP patients. A systematic review investigated the prevalence of MC and reported a median prevalence of 43% in CLBP patients and 6% in a non-clinical population [4]. Prevalence generally increases with age and peaks in the 60s [5]. Accumulating evidence shows that CLBP patients with DDD and MC are different from DDD patients without MC [6]. Patients with MC report a greater frequency and duration of low back pain (LBP) episodes, seek care more often, have a higher risk of a poor outcome, and have an ‘inflammatory pain pattern’ [4][7][8][9][10][11]. Larger lesions seem more painful and have a positive predictive value for pain of up to 100% [12][13]. Therefore, MC patients may in fact represent a clearly defined subpopulation of DDD patients. However, the effects of discal cell therapy at spinal levels of MC remain unknown.
Modic Changes
Vertebral bone marrow lesions adjacent to degenerated discs were first described by Assheuer et al. in 1987 [14] and later coined by Modic et al. in 1988 [15]. Three interconvertible types of MC have been defined based on their appearance in T1-weighted and T2-weighted magnetic resonance imaging (MRI) (Figure 1) [15][16]. Histological data of MC patient bone marrow are sparse [14][15][17][18]. In Modic type 1 changes (MC1), fibrosis, granulation tissue, lymphocytic and neutrophilic infiltrations, increased frequency of adipocytes, necrotic adipocytes, and interstitial water have been reported [14][15][19]. In Modic type 2 changes (MC2), the red hematopoietic bone marrow is replaced by fatty bone marrow and can contain displaced disc tissue along with fibrotic tissue [14][15][20]. Trabecular bone in MC1 is thinned, possibly due to osteoclastic activity, and thickened in MC2 [14][15][17]. Modic type 3 changes (MC3) represent extensive sclerotic changes [15][17]. Increased numbers of peptidergic nerve endings were found in MC1 and to a lesser extent in MC2 [18][19]. This may relate to the high specificity of MC for pain in discography [13][20].
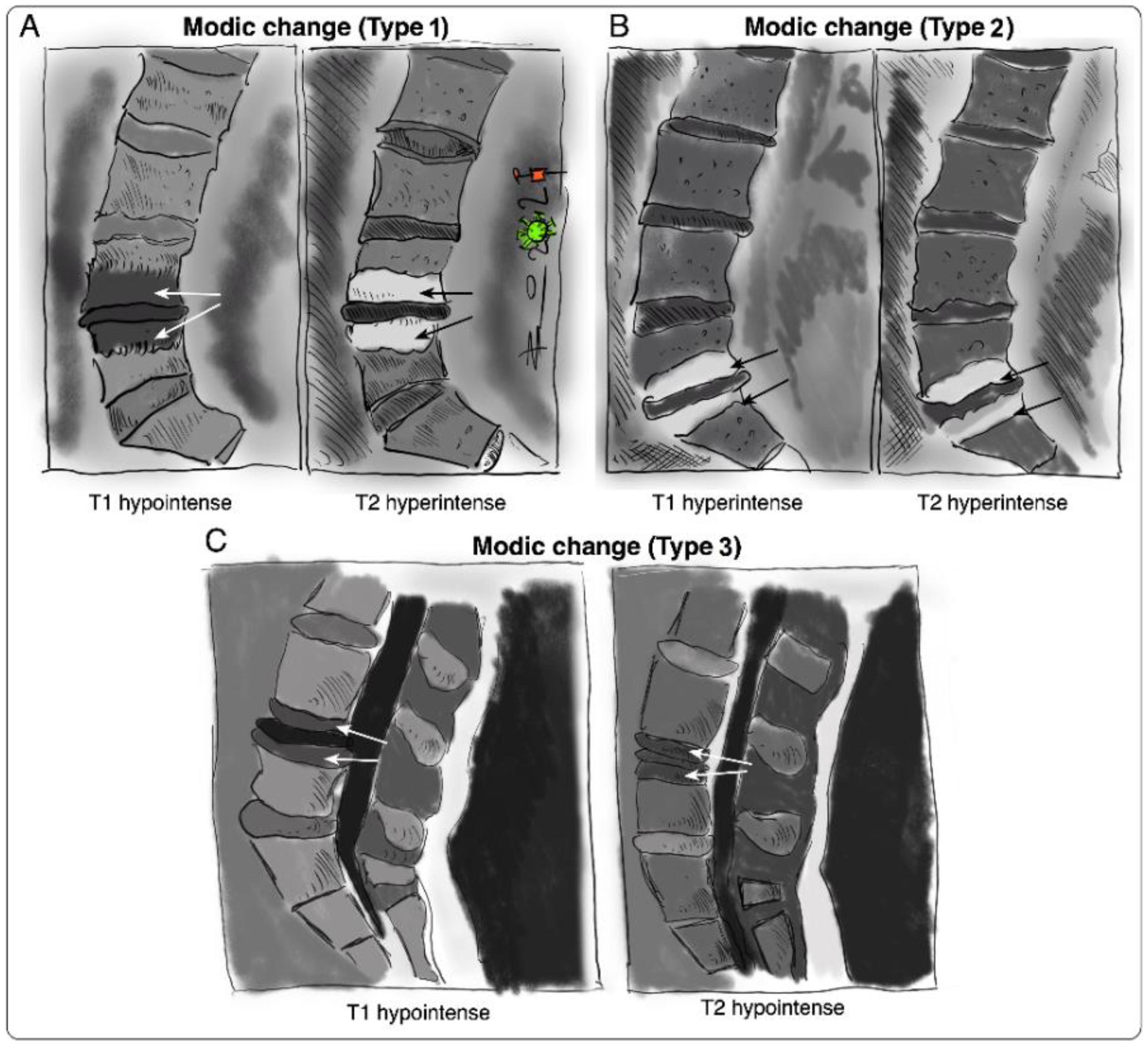 Figure 1. Sketches of intensity changes when scanning vertebral columns of human patients and classification of the three distinguishable MC according to T1- and T2-weighted sequences on MRI [15]. MC are classified into (A) MC type I, hypointense in T1 and hyperintense in T2, (B) MC type 2, hyperintense in T1 and T2, and (C) MC type 3, hypointense in T1 and T2.
Figure 1. Sketches of intensity changes when scanning vertebral columns of human patients and classification of the three distinguishable MC according to T1- and T2-weighted sequences on MRI [15]. MC are classified into (A) MC type I, hypointense in T1 and hyperintense in T2, (B) MC type 2, hyperintense in T1 and T2, and (C) MC type 3, hypointense in T1 and T2.The IVD and the vertebral endplate seem to play an important role in the pathomechanism of MC (Figure 2). MC only occur adjacent to degenerated discs and mostly develop simultaneously in the cranial and caudal vertebrae of the degenerated disc [16]. Progression of DD accompanies the progression or evolution of MC [21]. Vertebral endplate defects are strongly associated with MC and extensive endplate degeneration is a risk factor for the progression of DD and MC [21][22]. Endplate defects enhance the fluid flow between the disc and the bone marrow [23][24] and may provide a physical explanation for the inflammatory and pro-fibrotic cross-talk between the disc and the bone marrow observed in MC [25]. This cross-talk likely promotes MC development, thus representing an interesting treatment target. In vivo studies with mice, rats, and baboons confirm that disc injury can cause changes in the adjacent vertebrae, with alterations in marrow composition and remodeling of trabecular bone [26][27][28][29]. Analysis of human disc samples revealed increased expression of pro-inflammatory, pro-osteoclastic, and neurotrophic cytokines (Table 1) [19][30][31]. Notably, many of them can affect hematopoiesis and contribute to the hematopoietic changes observed in MC bone marrow [25].
Table 1. List of pro-inflammatory, pro-osteoclastic, and neurotrophic cytokines with elevated expression levels in ‘MC discs’.
| MC Type | Pro-Inflammatory | Pro-Osteoclastic | Neurotrophic |
|---|---|---|---|
| MC1 | CCL2, IL-6, IL-8, PGE2 | OSCAR | NTRK1 |
| MC2 | CCL2, CXCL5, GM-CSF, IL-1β, M-CSF | RANKL, RUNX1, RUNX2 | NTRK1 |
CCL2, C-C motif chemokine ligand 2; CXCL5, C-X-C motif chemokine ligand 5; GM-CSF, granulocyte-macrophage colony-stimulating factor; IL-1β, interleukin-1β; IL-6, interleukin-6; IL-8, interleukin-8; M-CSF, macrophage colony-stimulating factor; NTRK1, neurotrophic receptor tyrosine kinase 1; OSCAR, osteoclast-associated Ig-like receptor; PGE2, prostaglandin E2; RANKL, tumor necrosis factor superfamily member 11; RUNX1, runt-related transcription factor 1; RUNX2, runt-related transcription factor 2.
Despite an increasing understanding of the molecular and cellular changes in MC bone marrow and discs, the etiology of MC remains largely unknown. Autoinflammation against disc material and occult disc infection are both supported by clinical and experimental studies [30][31][32][33][34][35][36][37]. While infectious MC may be treated with antibiotics [36][37], no approved treatment or treatment consensus exists for autoinflammatory MC. Standard treatments for CLBP are generally less effective in MC1 patients [8][38]. Treatment attempts with intradiscal steroids, bisphosphonates, and tumor necrosis factor alpha (TNF-α) inhibitors to control inflammation had limited short-term efficacy, and lacked anatomical or biological specificity [39][40][41][42][43][44]. Spinal fusion surgery may relieve pain but can have serious risks besides those of surgery and anesthesia [45].
In summary, clinical and experimental data suggest that disc inflammation can affect the adjacent bone marrow via an enhanced cross-talk through damaged endplates. Therefore, suppression of discal inflammation might represent a promising treatment strategy to protect the bone marrow from the vicious cross-talk with the inflammatory disc.
 Figure 2. Schematic illustration of possible causes of pain and inflammation in ‘MC discs’, comparing a ‘healthy disc’ (on the left side) to a ‘Modic disc’ (on the right side). Note that the central role is given to the CEP: CEP damage possibly enables inflammation in the adjacent vertebrae, triggering a cross-talk to inflammatory cells. Ingrowth of nerve endings into the IVD might be responsible for pain development. Increased osteoclast activity might be responsible for the inflammatory trabecular bone resorption observed in MC1 [14][15][17]. MSCs in the bone marrow adjacent to ‘MC1 discs’ have a pro-fibrotic phenotype [46], possibly due to the pro-fibrotic and pro-inflammatory cross-talk with the ‘MC1 disc’.
Figure 2. Schematic illustration of possible causes of pain and inflammation in ‘MC discs’, comparing a ‘healthy disc’ (on the left side) to a ‘Modic disc’ (on the right side). Note that the central role is given to the CEP: CEP damage possibly enables inflammation in the adjacent vertebrae, triggering a cross-talk to inflammatory cells. Ingrowth of nerve endings into the IVD might be responsible for pain development. Increased osteoclast activity might be responsible for the inflammatory trabecular bone resorption observed in MC1 [14][15][17]. MSCs in the bone marrow adjacent to ‘MC1 discs’ have a pro-fibrotic phenotype [46], possibly due to the pro-fibrotic and pro-inflammatory cross-talk with the ‘MC1 disc’.2. Mesenchymal Stem Cell (MSC) Therapy for Degenerative Disc Disease (DDD)
Mesenchymal stem cells (MSCs) have been used in numerous clinical trials for DDD independent of concomitant MC during the past decade. Clinical trials for DDD using MSCs have recently been reviewed [1]. MSCs are an attractive option for cell therapies because of their self-renewal capacity, ease of isolation, multi-lineage differentiation potential, engraftment capacity, safety profile, and immunomodulatory properties. MSC treatments have been shown to be safe and well-tolerated, with no reported severe adverse events but with occasional mild pain-related adverse events [1][47][48]. Previous studies observed no host immune rejection against allogeneic MSCs, indicating that allogeneic MSCs might avoid immunogenic reactions in humans [1][49][50]. Although concerns about MSC-associated tumorigenesis, osteophyte formation, infection, and immune rejections are justified, none of these safety concerns have been confirmed in completed clinical trials on MSC injections in DDD patients.
The underlying concept of regenerative treatment approaches in DDD assumes that MSC injection reconstitutes the healthy disc anatomy and thereby restores the normal functioning of the motion segment [51]. The successful restoration of a functionally impaired motion segment could prevent the development of degenerative spinal pathologies [47][48][51]. The chance to disrupt the degenerative cascade led to the conduction of multiple clinical trials, investigating the use of intradiscal MSC injections to treat DDD [1]. Most completed clinical trials focused on IVD regeneration and the safety profile of MSC injections. Additionally, pain relief and disability-related improvements have been frequently investigated yet controlling disc inflammation has not been a focus of these trials. Despite tremendous efforts, substantial IVD regeneration could not be shown in any of the completed clinical trials on intradiscal MSC injections in DDD patients, besides occasional IVD rehydration and deceleration of degenerative processes [1]. Interestingly, DDD patients treated with intradiscal MSC injections experienced substantial pain relief and showed significant disability-related improvements in a group of responders [1][50][52]. These findings raised the question of whether IVD regeneration is essential for achieving favorable therapy outcomes and indicated that the analgesic effect of the MSC injections may be due to a regeneration-independent mode of action.
The multifactorial etiology of DDD could manifest in various sources of discogenic pain. The key processes of DDD that are related to discogenic pain have been reviewed elsewhere [49]. In summary, inflammation, an acidic IVD microenvironment, nerve ingrowth, and endplate damage were found to be closely linked to discogenic pain in DDD [53][49][50][52][54][55][56]. All these features are characteristics of MC, making MC one of the DDD-associated findings with the highest pain specificity [20][57]. These sources of discogenic LBP could potentially be targeted by the broad mode of action of MSCs, including the secretion of immunomodulatory factors, multi-lineage differentiation potential, and the promotion of cell survival [58][59].
In order to assess whether MSCs should be considered for ‘MC discs’, the regenerative and immunomodulatory mode of action of MSCs and their contribution to pain relief will be reviewed.
2.1. Regenerative Mode of Action
Structural damage of the IVD and cartilage endplate (CEP) might contribute to functional impairment of the vertebral motion segment, which in turn could result in painful discal inflammation [48][51]. Targeting the underlying biomechanical issue might alleviate or even prevent painful vertebral bone marrow inflammation and subsequent degenerative processes. MSCs can promote IVD regeneration by various mechanisms. Their ability to proliferate and differentiate into chondrocytes [60][61][62][63][64] could allow them to replace damaged IVD cells (IVDCs), thereby supporting chondrogenesis [65]. Additionally, MSCs can contribute to IVD regeneration by the de novo synthesis of the extracellular matrix (ECM) [66]. Paracrine secretion of anabolic growth factors, anti-catabolic factors, and immunomodulatory cytokines by MSCs influences the survival and function of resident IVDCs [66] and renders MSCs promising candidates for inducing IVD regeneration [61][66][67][68][69][70].
The cross-talk between MSCs and IVDCs was shown to downregulate the gene expression of pro-inflammatory cytokines in IVDCs and to significantly increase their insoluble collagen synthesis and proliferation rate in vitro [68]. Furthermore, the co-culture of MSCs with nucleus pulposus cells (NPCs) was shown to protect NPCs against compression-induced apoptosis by reducing the concentration of reactive oxygen species and maintaining mitochondrial integrity [71]. On the contrary, the cross-talk between MSCs and IVDCs did not lead to the significantly increased synthesis of insoluble collagen by MSCs, but clearly induced the gene expression of various growth factors [68]. The paracrine secretion of these growth factors might have led to the increased proliferation and collagen synthesis of IVDCs.
Unfortunately, in vitro studies are incapable of mimicking the complex IVD microenvironment. The hostile IVD microenvironment likely impairs the regenerative potential of MSCs [52]. To enable MSCs to contribute to substantial IVD regeneration, they must be adapted to or protected from the harsh IVD microenvironment, including nutrient deprivation, hypoxia, acidic pH, high osmolarity, and a combination of inflammatory cytokines [72][62][73][74]. The use of MSC licensing strategies and biomaterial scaffolds may help to improve the survivability and the therapeutic potential of MSCs. Modulation of the inflammatory environment before attempting to regenerate the IVD and CEP using MSCs might be necessary. A non-inflammatory IVD microenvironment is more likely to support larger numbers of chondrogenic MSCs, which are needed to induce regeneration [52][75].
The following section summarizes the characteristics of the IVD microenvironment, which were discussed in a comprehensive review by Vadalà et al. [76]. The impact of the harsh IVD microenvironment on IVDCs and MSCs and its relevance in MC will be discussed.
2.1.1. Nutrient and Oxygen Deficiency
The avascular nature of IVDs creates a hypoxic microenvironment with limited nutrient availability. Hypoxia (2–5% O2) and low glucose (1 mg/mL) were found to have positive effects on MSC-mediated IVD regeneration. MSCs cultured under hypoxia not only grew significantly faster than MSCs cultured under normoxia, but also had increased expression of genes associated with ECM assembly and improved differentiation potential [73][77][78][79][80]. The viability and proliferation of MSCs were maintained at IVD-like low glucose levels, whilst ECM biosynthesis was significantly enhanced [74][81]. Similarly, hypoxia supports the survival of NPCs and significantly enhances ECM biosynthesis and NPC proliferation [82][83][84][85]. Nutrients and oxygen are transported in blood vessels to the CEP and small capillaries supply nutrients through the CEP to the CEP/annulus fibrosus (AF) interface [86]. The nutrient supply of IVDCs then depends on diffusion from the CEP/AF interface into the IVD [86]. Endplate calcification increases with ageing and progression of DD and likely limits the diffusion of nutrients as the capillaries can no longer penetrate the endplate or are damaged as a result of the calcification [25][74][87]. Endplate defects are frequently seen in MC and have been shown to be responsible for substantial changes in diffusion between the IVDs and adjacent vertebral bodies [24]. No data on nutrient and oxygen concentrations in ‘MC discs’ have been published, but it can be speculated that the increased diffusion through damaged endplates increases the nutrient concentration and oxygen tension in ‘MC discs’, with unknown consequences for IVDC behavior and intradiscally injected MSCs.
2.1.2. Acidity
Non-degenerated IVDs have a pH between 7.1 and 7.4 but the pH can drop to 6.8 in mild DD and can reach values of 6.2 in severe DD [88][89]. The proliferation rate and viability of MSCs and NPCs decrease with increasing acidity [90]. Furthermore, an acidic pH stimulates NPCs to increase the secretion of pro-inflammatory cytokines, nerve growth factors, and catabolic enzymes [91][92][93]. The pH in ‘MC1 discs’ has not yet been investigated. Endplate leakage enhances the fluid flow between the IVD and the bone marrow, thus likely facilitating the efflux of acidic metabolites into the adjacent bone marrow. A low pH can lower the threshold for the activation of sensory nerve fibers through acid-sensing sodium channels and is hence directly linked to nociceptive pain [94]. This might be relevant in MC, because more sensory nerve fibers were found in the bone marrow close to the endplates [18][19].
2.1.3. Hyperosmolarity
The IVD has a hyperosmolar environment. In non-degenerated IVDs, osmolarity ranges between 430 and 500 mOsm/L [95] but steadily declines with the progression of DD due to loss of proteoglycans [95]. A hyperosmolar culture condition (485 mOsm/L) significantly decreases the gene expression of aggrecan and collagen-1 and decelerates MSC proliferation compared to standard cell culture conditions (280 mOsm/L) [81]. Therefore, reduced hyperosmolarity in degenerated IVDs might be beneficial for ECM deposition and the proliferation rate of intradiscally injected MSCs. Osmolarity in ‘MC discs’ has not been investigated; thus, the effect of osmolarity on intradiscally injected MSCs in ‘MC discs’ remains unknown.
In summary, it is challenging to restore a healthy IVD microenvironment by addressing single components of the complex network of cytokines, growth factors, catabolic enzymes, and neurotrophic factors found in degenerated IVDs [96]. An adaptable multimodal therapeutic approach might be needed to suppress the discal inflammation and to restore a healthy IVD microenvironment.
2.2. Immunomodulatory Mode of Action
The exceptional potential of MSCs to modulate a broad range of immune cells makes them an interesting candidate for the treatment of inflammatory disorders. The impact of MSCs on the functional properties of various cells from the innate and adaptive immune system has been thoroughly reviewed elsewhere [87][97]. In summary, MSCs can modulate immune cells through a paracrine mode of action. The secretion of immunomodulatory factors, including indoleamine 2,3-dioxygenase (IDO), TNF-α-inducible protein 6 (TSG-6), PGE2, IL-10, and transforming growth factor-beta (TGF-β), has distinct effects on various cell types. MSCs can regulate the antibody secretion of B cells, suppress T cell activation and proliferation, and prevent the activation of neutrophils [97]. Furthermore, MSCs can inhibit the maturation of dendritic cells and polarize macrophages towards immunomodulatory M2 macrophages. It has been shown that macrophages and other leukocytes [96] can infiltrate contained IVDs during degeneration and the number of infiltrated macrophages positively correlated with the progression of DD [91][98]. This might indicate the importance of MSCs in modulating a broad range of immune cells to resolve discal inflammation. Besides the secretion of immunomodulatory factors, MSCs interact with immune cells via cell–cell contact, mitochondrial transfer, and extracellular vesicles [1][87][92][93][99]. Regarding intradiscal MSC therapy, it is important that the immunomodulatory action of MSCs is not limited to leukocytes but also affects disc cells.
The secretion of inflammatory factors by IVD cells and infiltrating immune cells not only shifts the balance between anabolic and catabolic processes towards ECM degradation but also promotes the secretion of neurotrophic factors by IVD cells, ultimately leading to nerve ingrowth and discogenic pain [53][96][98][100][101][102][103]. Interestingly, co-culture of MSCs with degenerative IVDCs significantly downregulated the gene expression of pro-inflammatory cytokines (interleukin-1α (IL-1α), IL-1β, IL-6, TNF-α) [68]. Moreover, the co-culture of MSCs with degenerative NPCs significantly upregulated the gene expression of various growth factors (epidermal growth factor (EGF), insulin-like growth factor-1 (IGF-1), osteogenic protein-1 (OP-1), growth differentiation factor-7 (GDF-7), and TGF-β in MSCs. This research demonstrated the immunomodulatory potential of MSCs to modulate the degenerative IVDCs. To consider MSCs for the treatment of MC, MSCs must suppress the inflammation in the ‘MC disc’. Elevated levels of inflammatory molecules including TNF-α, IL-1β, IL-6, interferon-gamma (IFN-γ), and interleukin-17 (IL-17) are common findings in degenerating IVDs [53][91][104][105][106][107]. The effect of these elevated inflammatory molecules on IVDCs was investigated by Gabr et al. [107]. IVDCs from patients undergoing surgery for DD or scoliosis were stimulated with IL-17 in combination with IFN-γ or TNF-α. The stimulation of IVDCs significantly increased the secretion of inflammatory molecules (nitric oxide (NO), PGE2, IL-6, and intercellular adhesion molecule-1 (ICAM-1)), thereby indicating the potential of stimulated IVDCs to recruit immune cells to the IVD tissue [107].
2.3. Immunomodulatory vs. Regenerative Mode of Action in DDD
An ex vivo experimental study—using a bovine model of IVD degeneration—investigated the effect of a degenerative IVD microenvironment on the regenerative and immunomodulatory potential of human MSCs in co-culture with the bovine IVD [108]. No notable effect on ECM remodeling by MSCs was found, but evidence was presented for an immunomodulatory paracrine effect of MSCs, suggesting a predominant cytokine feedback loop between MSCs and disc cells [108]. In summary, the co-culture of human MSCs with bovine IVDs in an inflammatory environment led to the significant downregulation of bovine pro-inflammatory cytokines, including IL-6, IL-8, and TNF-α [108]. This research indicated that the immunomodulatory potential of MSCs might be more relevant than the regenerative capacity in an inflammatory IVD microenvironment. Furthermore, it raised the question as to what extent the regenerative capacity of MSCs contributed to the favorable outcomes of previous clinical trials on intradiscal MSC injections in DDD patients. In conclusion, the immunomodulatory potential of MSCs is an important factor in discal MSC therapy that deserves more attention.
This entry is adapted from the peer-reviewed paper 10.3390/ijms23052721
References
- Binch, A.L.A.; Fitzgerald, J.C.; Growney, E.A.; Barry, F. Cell-based strategies for IVD repair: Clinical progress and translational obstacles. Nat. Rev. Rheumatol. 2021, 17, 158–175.
- Cheung, K.M.C.; Karppinen, J.; Chan, D.; Ho, D.W.H.; Song, Y.-Q.; Sham, P.; Cheah, K.S.E.; Leong, J.C.Y.; Luk, K.D.K. Prevalence and Pattern of Lumbar Magnetic Resonance Imaging Changes in a Population Study of One Thousand Forty-Three Individuals. Spine 2009, 34, 934–940.
- Brinjikji, W.; Diehn, F.E.; Jarvik, J.G.; Carr, C.M.; Kallmes, D.F.; Murad, M.H.; Luetmer, P.H. MRI Findings of Disc Degeneration are More Prevalent in Adults with Low Back Pain than in Asymptomatic Controls: A Systematic Review and Meta-Analysis. Am. J. Neuroradiol. 2015, 36, 2394–2399.
- Jensen, T.S.; Karppinen, J.; Sorensen, J.S.; Niinimäki, J.; Leboeuf-Yde, C. Vertebral endplate signal changes (Modic change): A systematic literature review of prevalence and association with non-specific low back pain. Eur. Spine J. 2008, 17, 1407–1422.
- Tarukado, K.; Ono, T.; Tono, O.; Tanaka, H.; Ikuta, K.; Harimaya, K.; Doi, T. Does Modic Change Progresss with Age? Spine 2017, 42, 1805–1809.
- Kjaer, P.; Korsholm, L.; Bendix, T.; Sorensen, J.S.; Leboeuf-Yde, C. Modic changes and their associations with clinical findings. Eur. Spine J. 2006, 15, 1312–1319.
- Sørlie, A.; Moholdt, V.; Kvistad, K.A.; Nygaard, P.; Ingebrigtsen, T.; Iversen, T.; Kloster, R.; Solberg, T.K. Modic type I changes and recovery of back pain after lumbar microdiscectomy. Eur. Spine J. 2012, 21, 2252–2258.
- Jensen, O.K.; Nielsen, C.V.; Sørensen, J.S.; Stengaard-Pedersen, K. Type 1 Modic changes was a significant risk factor for 1-year outcome in sick-listed low back pain patients: A nested cohort study using magnetic resonance imaging of the lumbar spine. Spine J. 2014, 14, 2568–2581.
- Schistad, E.I.; Espeland, A.; Rygh, L.J.; Røe, C.; Gjerstad, J.; Schistad, E.I.; Espeland, A.; Rygh, L.J.; Røe, C.; Gjerstad, J. The association between Modic changes and pain during 1-year follow-up in patients with lumbar radicular pain. Skelet. Radiol. 2014, 43, 1271–1279.
- Chung, C.B.; Berg, B.C.V.; Tavernier, T.; Cotten, A.; Laredo, J.-D.; Vallee, C.; Malghem, J. End plate marrow changes in the asymptomatic lumbosacral spine: Frequency, distribution and correlation with age and degenerative changes. Skelet. Radiol. 2004, 33, 399–404.
- Bailly, F.; Maigne, J.-Y.; Genevay, S.; Marty, M.; Gandjbakhch, F.; Rozenberg, S.; Foltz, V. Inflammatory pain pattern and pain with lumbar extension associated with Modic 1 changes on MRI: A prospective case–control study of 120 patients. Eur. Spine J. 2013, 23, 493–497.
- Järvinen, J.; Karppinen, J.; Niinimäki, J.; Haapea, M.; Grönblad, M.; Luoma, K.; Rinne, E. Association between changes in lumbar Modic changes and low back symptoms over a two-year period. BMC Musculoskelet. Disord. 2015, 16, 98.
- Weishaupt, D.; Zanetti, M.; Hodler, J.; Min, K.; Fuchs, B.; Pfirrmann, C.W.; Boos, N. Painful Lumbar Disk Derangement: Relevance of Endplate Abnormalities at MR Imaging. Radiology 2001, 218, 420–427.
- Assheuer, J.; Lenz, G.; Lenz, W.; Gottschlich, K.W.; Schulitz, K.P. Fat/Water Separation in the NMR Tomogram. The Imaging of Bone Marrow Reactions in Degenerative Intervertebral Disk Changes. Rofo 1987, 147, 58–63.
- Modic, M.T.; Steinberg, P.M.; Ross, J.S.; Masaryk, T.J.; Carter, J.R. Degenerative disk disease: Assessment of changes in vertebral body marrow with MR imaging. Radiology 1988, 166, 193–199.
- Jensen, T.S.; Bendix, T.; Sorensen, J.S.; Manniche, C.; Korsholm, L.; Kjaer, P. Characteristics and natural course of vertebral endplate signal (Modic) changes in the Danish general population. BMC Musculoskelet. Disord. 2009, 10, 81.
- Perilli, E.; Parkinson, I.H.; Truong, L.-H.; Chong, K.C.; Fazzalari, N.L.; Osti, O.L. Modic (endplate) changes in the lumbar spine: Bone micro-architecture and remodelling. Eur. Spine J. 2015, 24, 1926–1934.
- Fields, A.J.; Liebenberg, E.C.; Lotz, J.C. Innervation of pathologies in the lumbar vertebral end plate and intervertebral disc. Spine J. 2014, 14, 513–521.
- Dudli, S.; Sing, D.C.; Hu, S.S.; Berven, S.H.; Burch, S.; Deviren, V.; Cheng, I.; Tay, B.K.B.; Alamin, T.F.; Ith, M.A.M.; et al. ISSLS PRIZE IN BASIC SCIENCE 2017: Intervertebral disc/bone marrow cross-talk with Modic changes. Eur. Spine J. 2017, 26, 1362–1373.
- Lotz, J.C.; Fields, A.; Liebenberg, E.C. The Role of the Vertebral End Plate in Low Back Pain. Glob. Spine J. 2013, 3, 153–163.
- Ohtori, S.; Inoue, G.; Ito, T.; Koshi, T.; Ozawa, T.; Doya, H.; Saito, T.; Moriya, H.; Takahashi, K. Tumor Necrosis Factor-Immunoreactive Cells and PGP 9.5-Immunoreactive Nerve Fibers in Vertebral Endplates of Patients With Discogenic Low Back Pain and Modic Type 1 or Type 2 Changes on MRI. Spine 2006, 31, 1026–1031.
- Farshad-Amacker, N.A.; Hughes, A.; Herzog, R.J.; Seifert, B.; Farshad, M. The intervertebral disc, the endplates and the vertebral bone marrow as a unit in the process of degeneration. Eur. Radiol. 2017, 27, 2507–2520.
- Määttä, J.H.; Rade, M.; Freidin, M.B.; Airaksinen, O.; Karppinen, J.; Williams, F.M.K. Strong association between vertebral endplate defect and Modic change in the general population. Sci. Rep. 2018, 8, 16630.
- Ferguson, S.J.; Ito, K.; Nolte, L.-P. Fluid flow and convective transport of solutes within the intervertebral disc. J. Biomech. 2003, 37, 213–221.
- Rajasekaran, S.; Babu, J.N.; Arun, R.; Armstrong, B.R.W.; Shetty, A.; Murugan, S. ISSLS Prize Winner: A Study of Diffusion in Human Lumbar Discs: A Serial Magnetic Resonance Imaging Study Documenting the Influence of the Endplate on Diffusion in Normal and Degenerate Discs. Spine 2004, 29, 2654–2667.
- Malinin, T.; Brown, M.D. Changes in Vertebral Bodies Adjacent to Acutely Narrowed Intervertebral Discs: Observations in Baboons. Spine 2007, 32, E603–E607.
- Ulrich, J.A.; Liebenberg, E.C.; Thuillier, D.U.; Lotz, J.C. ISSLS Prize Winner: Repeated Disc Injury Causes Persistent Inflammation. Spine 2007, 32, 2812–2819.
- Papuga, M.O.; Proulx, S.; Kwok, E.; You, Z.; Rubery, P.T.; Dougherty, P.E.; Hilton, M.; Awad, H.; Schwarz, E.M. Chronic axial compression of the mouse tail segment induces MRI bone marrow edema changes that correlate with increased marrow vasculature and cellularity. J. Orthop. Res. 2010, 28, 1220–1228.
- Moore, R.J.; Vernon-Roberts, B.; Osti, O.L.; Fraser, R.D. Remodeling of Vertebral Bone After Outer Anular Injury in Sheep. Spine 1996, 21, 936–940.
- Torkki, M.; Majuri, M.-L.; Wolff, H.; Koskelainen, T.; Haapea, M.; Niinimäki, J.; Alenius, H.; Lotz, J.; Karppinen, J. Osteoclast activators are elevated in intervertebral disks with Modic changes among patients operated for herniated nucleus pulposus. Eur. Spine J. 2016, 25, 207–216.
- Burke, J.; Watson, R.; McCormack, D.; Fitzpatrick, J.M.; Stack, J.; Walsh, M. Modic changes are associated with increased disc inflammatory mediator production. Spine J. 2002, 2, 3–4.
- Dudli, S.; Liebenberg, E.; Magnitsky, S.; Lu, B.; Lauricella, M.; Lotz, J.C. Modic type 1 change is an autoimmune response that requires a proinflammatory milieu provided by the ‘Modic disc’. Spine J. 2018, 18, 831–844.
- Dudli, S.; Liebenberg, E.; Magnitsky, S.; Miller, S.; Demir-Deviren, S.; Lotz, J.C. Propionibacterium acnesinfected intervertebral discs cause vertebral bone marrow lesions consistent with Modic changes. J. Orthop. Res. 2016, 34, 1447–1455.
- Geiss, A.; Larsson, K.; Junevik, K.; Rydevik, B.; Olmarker, K. Autologous nucleus pulposus primes T cells to develop into interleukin-4-producing effector cells: An experimental study on the autoimmune properties of nucleus pulposus. J. Orthop. Res. 2008, 27, 97–103.
- Gertzbein, S.D.; Tait, J.H.; Devlin, S.R. The stimulation of lymphocytes by nucleus pulposus in patients with degenerative disk disease of the lumbar spine. Clin. Orthop. Relat. Res. 1976, 123, 149–154.
- Capossela, S.; Schläfli, P.; Bertolo, A.; Janner, T.; Stadler, B.M.; Pötzel, T.; Baur, M.; Stoyanov, J.V. Degenerated human intervertebral discs contain autoantibodies against extracellular matrix proteins. Eur. Cells Mater. 2014, 27, 251–263.
- Capoor, M.N.; Ruzicka, F.; Schmitz, J.E.; James, G.A.; Machackova, T.; Jancalek, R.; Smrcka, M.; Lipina, R.; Ahmed, F.S.; Alamin, T.F.; et al. Propionibacterium acnes biofilm is present in intervertebral discs of patients undergoing microdiscectomy. PLoS ONE 2017, 12, e0174518.
- Gilligan, C.J.; Cohen, S.P.; Fischetti, V.A.; Hirsch, J.A.; Czaplewski, L.G. Chronic low back pain, bacterial infection and treatment with antibiotics. Spine J. 2021, 21, 903–914.
- Manniche, C.; Hall, G.M. Chronic low back pain, Modic changes and low-grade virulent infection: Efficacy of antibiotic treatment. Futur. Sci. OA 2021, 7, FSO703.
- Annen, M.; Peterson, C.; Leemann, S.; Schmid, C.; Anklin, B.; Humphreys, B.K. Comparison of Outcomes in MRI Confirmed Lumbar Disc Herniation Patients With and Without Modic Changes Treated With High Velocity, Low Amplitude Spinal Manipulation. J. Manip. Physiol. Ther. 2016, 39, 200–209.
- Fayad, F.; Lefevre-Colau, M.-M.; Rannou, F.; Quintero, N.; Nys, A.; Macé, Y.; Poiraudeau, S.; Drapé, J.L.; Revel, M. Relation of inflammatory modic changes to intradiscal steroid injection outcome in chronic low back pain. Eur. Spine J. 2007, 16, 925–931.
- Cao, P.; Jiang, L.; Zhuang, C.; Yang, Y.; Zhang, Z.; Chen, W.; Zheng, T. Intradiscal injection therapy for degenerative chronic discogenic low back pain with end plate Modic changes. Spine J. 2011, 11, 100–106.
- Beaudreuil, J.; Dieude, P.; Poiraudeau, S.; Revel, M. Disabling chronic low back pain with Modic type 1 MRI signal: Acute reduction in pain with intradiscal corticotherapy. Ann. Phys. Rehabil. Med. 2012, 55, 139–147.
- Koivisto, K.; Järvinen, J.; Karppinen, J.; Haapea, M.; Paananen, M.; Kyllönen, E.; Tervonen, O.; Niinimäki, J. The effect of zoledronic acid on type and volume of Modic changes among patients with low back pain. BMC Musculoskelet. Disord. 2017, 18, 274.
- Koivisto, K.; Kyllönen, E.; Haapea, M.; Niinimäki, J.; Sundqvist, K.; Pehkonen, T.; Seitsalo, S.; Tervonen, O.; Karppinen, J. Efficacy of zoledronic acid for chronic low back pain associated with Modic changes in magnetic resonance imaging. BMC Musculoskelet. Disord. 2014, 15, 64.
- Korhonen, T.; Karppinen, J.; Paimela, L.; Malmivaara, A.; Lindgren, K.-A.; Bowman, C.; Hammond, A.; Kirkham, B.; Järvinen, S.; Niinimäki, J.; et al. The Treatment of Disc Herniation-Induced Sciatica With Infliximab: One-Year Follow-up Results of FIRST II, a Randomized Controlled Trial. Spine 2006, 31, 2759–2766.
- Elabd, C.; Centeno, C.J.; Schultz, J.R.; Lutz, G.; Ichim, T.; Silva, F.J. Intra-discal injection of autologous, hypoxic cultured bone marrow-derived mesenchymal stem cells in five patients with chronic lower back pain: A long-term safety and feasibility study. J. Transl. Med. 2016, 14, 253.
- Comella, K.; Silbert, R.; Parlo, M. Effects of the intradiscal implantation of stromal vascular fraction plus platelet rich plasma in patients with degenerative disc disease. J. Transl. Med. 2017, 15, 12.
- Ryan, J.M.; Barry, F.P.; Murphy, J.M.; Mahon, B.P. Mesenchymal stem cells avoid allogeneic rejection. J. Inflamm. 2005, 2, 8.
- Noriega, D.C.; Ardura, F.; Hernández-Ramajo, R.; Martín-Ferrero, M.Á.; Sánchez-Lite, I.; Toribio, B.; Alberca, M.; García, V.; Moraleda, J.M.; Sánchez, A.; et al. Intervertebral Disc Repair by Allogeneic Mesenchymal Bone Marrow Cells: A Randomized Controlled Trial. Transplantation 2017, 101, 1945–1951.
- Akeda, K.; Ohishi, K.; Masuda, K.; Bae, W.C.; Takegami, N.; Yamada, J.; Nakamura, T.; Sakakibara, T.; Kasai, Y.; Sudo, A. Intradiscal Injection of Autologous Platelet-Rich Plasma Releasate to Treat Discogenic Low Back Pain: A Preliminary Clinical Trial. Asian Spine J. 2017, 11, 380–389.
- Fujii, K.; Yamazaki, M.; Kang, J.D.; Risbud, M.V.; Cho, S.K.; Qureshi, S.A.; Hecht, A.C.; Iatridis, J.C. Discogenic Back Pain: Literature Review of Definition, Diagnosis, and Treatment. JBMR Plus 2019, 3, e10180.
- Gjefsen, E.; Bråten, L.C.H.; Goll, G.L.; Wigemyr, M.; Bolstad, N.; Valberg, M.; Schistad, E.I.; Marchand, G.H.; Granviken, F.; Selmer, K.K.; et al. The effect of infliximab in patients with chronic low back pain and Modic changes (the BackToBasic study): Study protocol of a randomized, double blind, placebo-controlled, multicenter trial. BMC Musculoskelet. Disord. 2020, 21, 698.
- Sakai, D.; Andersson, G.B.J. Stem cell therapy for intervertebral disc regeneration: Obstacles and solutions. Nat. Rev. Rheumatol. 2015, 11, 243–256.
- Roughley, P.J. Biology of Intervertebral Disc Aging and Degeneration. Spine 2004, 29, 2691–2699.
- Sakai, D. Future perspectives of cell-based therapy for intervertebral disc disease. Eur. Spine J. 2008, 17, 452–458.
- Lai, A.; Moon, A.; Purmessur, D.; Skovrlj, B.; Laudier, D.M.; Winkelstein, B.A.; Cho, S.K.; Hecht, A.C.; Iatridis, J.C. Annular puncture with tumor necrosis factor-alpha injection enhances painful behavior with disc degeneration in vivo. Spine J. 2015, 16, 420–431.
- Aoki, Y.; Ohtori, S.; Ino, H.; Douya, H.; Ozawa, T.; Saito, T.; Moriya, H.; Takahashi, K. Disc Inflammation Potentially Promotes Axonal Regeneration of Dorsal Root Ganglion Neurons Innervating Lumbar Intervertebral Disc in Rats. Spine 2004, 29, 2621–2626.
- Krock, E.; Rosenzweig, D.H.; Chabot-Doré, A.-J.; Jarzem, P.; Weber, M.H.; Ouellet, J.A.; Stone, L.S.; Haglund, L. Painful, degenerating intervertebral discs up-regulate neurite sprouting and CGRP through nociceptive factors. J. Cell. Mol. Med. 2014, 18, 1213–1225.
- Peng, Y.; Lv, F.-J. Symptomatic versus Asymptomatic Intervertebral Disc Degeneration: Is Inflammation the Key? Crit. Rev. Eukaryot. Gene Expr. 2015, 25, 13–21.
- Loibl, M.; Wuertz-Kozak, K.; Vadala, G.; Lang, S.; Fairbank, J.; Urban, J.P. Controversies in regenerative medicine: Should intervertebral disc degeneration be treated with mesenchymal stem cells? JOR Spine 2019, 2, e1043.
- Saukkonen, J.; Määttä, J.; Oura, P.; Kyllönen, E.; Tervonen, O.; Niinimäki, J.; Auvinen, J.; Karppinen, J. Association Between Modic Changes and Low Back Pain in Middle Age: A Northern Finland Birth Cohort Study. Spine 2020, 45, 1360–1367.
- Spees, J.L.; Lee, R.H.; Gregory, C.A. Mechanisms of mesenchymal stem/stromal cell function. Stem Cell Res. Ther. 2016, 7, 125.
- Mukhamedshina, Y.O.; Gracheva, O.A.; Mukhutdinova, D.M.; Chelyshev, Y.A.; Rizvanov, A. Mesenchymal stem cells and the neuronal microenvironment in the area of spinal cord injury. Neural Regen. Res. 2019, 14, 227–237.
- Frauchiger, D.A.; Heeb, S.R.; May, R.D.; Wöltje, M.; Benneker, L.M.; Gantenbein, B. Differentiation of MSC and annulus fibrosus cells on genetically engineered silk fleece-membrane-composites enriched for GDF-6 or TGF-β3. J. Orthop. Res. 2017, 36, 1324–1333.
- Clarke, L.; McConnell, J.C.; Sherratt, M.J.; Derby, B.; Richardson, S.M.; Hoyland, J. Growth differentiation factor 6 and transforming growth factor-beta differentially mediate mesenchymal stem cell differentiation, composition, and micromechanical properties of nucleus pulposus constructs. Arthritis Res. Ther. 2014, 16, R67.
- Dai, J.; Wang, H.; Liu, G.; Xu, Z.; Li, F.; Fang, H. Dynamic compression and co-culture with nucleus pulposus cells promotes proliferation and differentiation of adipose-derived mesenchymal stem cells. J. Biomech. 2014, 47, 966–972.
- Peroglio, M.; Eglin, D.; Benneker, L.M.; Alini, M.; Grad, S. Thermoreversible hyaluronan-based hydrogel supports in vitro and ex vivo disc-like differentiation of human mesenchymal stem cells. Spine J. 2013, 13, 1627–1639.
- Le Maitre, C.L.; Baird, P.; Freemont, A.J.; A Hoyland, J. An in vitro study investigating the survival and phenotype of mesenchymal stem cells following injection into nucleus pulposus tissue. Arthritis Res. Ther. 2009, 11, R20.
- Zha, K.; Li, X.; Yang, Z.; Tian, G.; Sun, Z.; Sui, X.; Dai, Y.; Liu, S.; Guo, Q. Heterogeneity of mesenchymal stem cells in cartilage regeneration: From characterization to application. NPJ Regen. Med. 2021, 6, 14.
- Richardson, S.M.; Kalamegam, G.; Pushparaj, P.N.; Matta, C.; Memic, A.; Khademhosseini, A.; Mobasheri, R.; Poletti, F.L.; Hoyland, J.A.; Mobasheri, A. Mesenchymal stem cells in regenerative medicine: Focus on articular cartilage and intervertebral disc regeneration. Methods 2016, 99, 69–80.
- Lyu, F.-J.; Cui, H.; Pan, H.; Cheung, K.M.C.; Cao, X.; Iatridis, J.C.; Zheng, Z. Painful intervertebral disc degeneration and inflammation: From laboratory evidence to clinical interventions. Bone Res. 2021, 9, 7.
- Vadalà, G.; Ambrosio, L.; Russo, F.; Papalia, R.; Denaro, V. Interaction between Mesenchymal Stem Cells and Intervertebral Disc Microenvironment: From Cell Therapy to Tissue Engineering. Stem Cells Int. 2019, 2019, 2376172.
- Baumgartner, L.; Arnhold, S.; Brixius, K.; Addicks, K.; Bloch, W. Human mesenchymal stem cells: Influence of oxygen pressure on proliferation and chondrogenic differentiation in fibrin gluein vitro. J. Biomed. Mater. Res. Part A 2009, 9999A, 930–940.
- Richardson, S.M.; Hoyland, J.A.; Mobasheri, R.; Csaki, C.; Shakibaei, M.; Mobasheri, A. Mesenchymal stem cells in regenerative medicine: Opportunities and challenges for articular cartilage and intervertebral disc tissue engineering. J. Cell. Physiol. 2010, 222, 23–32.
- Shim, E.-K.; Lee, J.-S.; Kim, D.-E.; Kim, S.K.; Jung, B.-J.; Choi, E.-Y.; Kim, C.-S. Autogenous Mesenchymal Stem Cells from the Vertebral Body Enhance Intervertebral Disc Regeneration via Paracrine Interaction: An in Vitro Pilot Study. Cell Transplant. 2016, 25, 1819–1832.
- Stoyanov, J.; Gantenbein-Ritter, B.; Bertolo, A.; Aebli, N.; Baur, M.; Alini, M.; Grad, S. Role of hypoxia and growth and differentiation factor-5 on differentiation of human mesenchymal stem cells towards intervertebral nucleus pulposus-like cells. Eur. Cells Mater. 2011, 21, 533–547.
- Gantenbein-Ritter, B.; Benneker, L.M.; Alini, M.; Grad, S. Differential response of human bone marrow stromal cells to either TGF-β1 or rhGDF-5. Eur. Spine J. 2010, 20, 962–971.
- Chen, S.; Zhao, L.; Deng, X.; Shi, D.; Wu, F.; Liang, H.; Huang, D.; Shao, Z. Mesenchymal Stem Cells Protect Nucleus Pulposus Cells from Compression-Induced Apoptosis by Inhibiting the Mitochondrial Pathway. Stem Cells Int. 2017, 2017, 9843120.
- Huang, Y.-C.; Leung, V.Y.; Lu, W.W.; Luk, K.D. The effects of microenvironment in mesenchymal stem cell–based regeneration of intervertebral disc. Spine J. 2013, 13, 352–362.
- Chiang, E.; Ma, H.; Wang, J.; Chang, M.; Liu, C.; Chen, T.; Hung, S. Use of Allogeneic Hypoxic Mesenchymal Stem Cells For Treating Disc Degeneration in Rabbits. J. Orthop. Res. 2019, 37, 1440–1450.
- Tsai, C.-C.; Chen, Y.-J.; Yew, T.-L.; Chen, L.-L.; Wang, J.-Y.; Chiu, C.-H.; Hung, S.-C. Hypoxia inhibits senescence and maintains mesenchymal stem cell properties through down-regulation of E2A-p21 by HIF-TWIST. Blood 2011, 117, 459–469.
- Basciano, L.; Nemos, C.; Foliguet, B.; de Isla, N.; de Carvalho, M.; Tran, N.; Dalloul, A. Long term culture of mesenchymal stem cells in hypoxia promotes a genetic program maintaining their undifferentiated and multipotent status. BMC Cell Biol. 2011, 12, 12.
- Grayson, W.L.; Zhao, F.; Bunnell, B.; Ma, T. Hypoxia enhances proliferation and tissue formation of human mesenchymal stem cells. Biochem. Biophys. Res. Commun. 2007, 358, 948–953.
- Liang, C.; Li, H.; Tao, Y.; Zhou, X.; Li, F.; Chen, G.; Chen, Q. Responses of human adipose-derived mesenchymal stem cells to chemical microenvironment of the intervertebral disc. J. Transl. Med. 2012, 10, 49.
- Wuertz, K.; Godburn, K.; Neidlinger-Wilke, C.; Urban, J.; Iatridis, J. Behavior of Mesenchymal Stem Cells in the Chemical Microenvironment of the Intervertebral Disc. Spine 2008, 33, 1843–1849.
- Gilbert, H.T.J.; Hodson, N.; Baird, P.; Richardson, S.; Hoyland, J.A. Acidic pH promotes intervertebral disc degeneration: Acid-sensing ion channel -3 as a potential therapeutic target. Sci. Rep. 2016, 6, 37360.
- Bischof, M.; Häckel, S.; Oberli, A.; Croft, A.; Oswald, K.; Albers, C.; Gantenbein, B.; Guerrero, J. Influence of Angiopoietin Treatment with Hypoxia and Normoxia on Human Intervertebral Disc Progenitor Cell’s Proliferation, Metabolic Activity, and Phenotype. Appl. Sci. 2021, 11, 7144.
- Chen, J.-W.; Li, B.; Yang, Y.-H.; Jiang, S.-D.; Jiang, L.-S. Significance of hypoxia in the physiological function of intervertebral disc cells. Crit. Rev. Eukaryot. Gene Expr. 2014, 24, 193–204.
- Feng, G.; Li, L.; Liu, H.; Song, Y.; Huang, F.; Tu, C.; Shen, B.; Gong, Q.; Li, T.; Zeng, J.; et al. Hypoxia differentially regulates human nucleus pulposus and annulus fibrosus cell extracellular matrix production in 3D scaffolds. Osteoarthr. Cartil. 2013, 21, 582–588.
- Liu, J.; Tao, H.; Wang, H.; Dong, F.; Zhang, R.; Li, J.; Ge, P.; Song, P.; Zhang, H.; Xu, P.; et al. Biological Behavior of Human Nucleus Pulposus Mesenchymal Stem Cells in Response to Changes in the Acidic Environment During Intervertebral Disc Degeneration. Stem Cells Dev. 2017, 26, 901–911.
- Su, Y.-S.; Sun, W.-H.; Chen, C.-C. Molecular mechanism of inflammatory pain. World J. Anesthesiol. 2014, 3, 71–81.
- Urban, J.P.G. The role of the physicochemical environment in determining disc cell behaviour. Biochem. Soc. Trans. 2002, 30, 858–863.
- Mwale, F.; Ciobanu, I.; Giannitsios, D.; Roughley, P.; Steffen, T.; Antoniou, J. Effect of Oxygen Levels on Proteoglycan Synthesis by Intervertebral Disc Cells. Spine 2011, 36, E131–E138.
- Grunhagen, T.; Shirazi-Adl, A.; Fairbank, J.C.; Urban, J.P. Intervertebral Disk Nutrition: A Review of Factors Influencing Concentrations of Nutrients and Metabolites. Orthop. Clin. N. Am. 2011, 42, 465–477.
- Vo, N.V.; Hartman, R.A.; Patil, P.R.; Risbud, M.V.; Kletsas, D.; Iatridis, J.; Hoyland, J.A.; Le Maitre, C.; Sowa, G.A.; Kang, J.D. Molecular mechanisms of biological aging in intervertebral discs. J. Orthop. Res. 2016, 34, 1289–1306.
- Wuertz, K.; Godburn, K.; Iatridis, J. MSC response to pH levels found in degenerating intervertebral discs. Biochem. Biophys. Res. Commun. 2009, 379, 824–829.
- Tsai, T.-T.; Danielson, K.G.; Guttapalli, A.; Oguz, E.; Albert, T.J.; Shapiro, I.M.; Risbud, M.V. TonEBP/OREBP Is a Regulator of Nucleus Pulposus Cell Function and Survival in the Intervertebral Disc. J. Biol. Chem. 2006, 281, 25416–25424.
- Risbud, M.V.; Shapiro, I.M. Role of cytokines in intervertebral disc degeneration: Pain and disc content. Nat. Rev. Rheumatol. 2013, 10, 44–56.
- Müller, L.; Tunger, A.; Wobus, M.; von Bonin, M.; Towers, R.; Bornhäuser, M.; Dazzi, F.; Wehner, R.; Schmitz, M. Immunomodulatory Properties of Mesenchymal Stromal Cells: An Update. Front. Cell Dev. Biol. 2021, 9, 637725.
- Joel, M.D.M.; Yuan, J.; Wang, J.; Yan, Y.; Qian, H.; Zhang, X.; Xu, W.; Mao, F. MSC: Immunoregulatory effects, roles on neutrophils and evolving clinical potentials. Am. J. Transl. Res. 2019, 11, 3890–3904.
- Yang, H.; Liu, B.; Liu, Y.; He, D.; Xing, Y.; An, Y.; Tian, W. Secreted Factors From Intervertebral Disc Cells and Infiltrating Macrophages Promote Degenerated Intervertebral Disc Catabolism. Spine 2019, 44, E520–E529.
- Shamji, M.F.; Setton, L.A.; Jarvis, W.; So, S.; Chen, J.; Jing, L.; Bullock, R.; Isaacs, R.E.; Brown, C.; Richardson, W. Pro-inflammatory cytokine expression profile in degenerative and herniated human intervertebral disc tissues. Arthritis Care Res. 2010, 62, 1974–1982.
- Wu, X.; Jiang, J.; Gu, Z.; Zhang, J.; Chen, Y.; Liu, X. Mesenchymal stromal cell therapies: Immunomodulatory properties and clinical progress. Stem Cell Res. Ther. 2020, 11, 345.
- De Castro, L.L.; Lopes-Pacheco, M.; Weiss, D.J.; Cruz, F.F.; Rocco, P.R.M. Current understanding of the immunosuppressive properties of mesenchymal stromal cells. Klin. Wochenschr. 2019, 97, 605–618.
- Wang, M.; Yuan, Q.; Xie, L. Mesenchymal Stem Cell-Based Immunomodulation: Properties and Clinical Application. Stem Cells Int. 2018, 2018, 3057624.
- Le Maitre, C.L.; Freemont, A.J.; Hoyland, J.A. The role of interleukin-1 in the pathogenesis of human Intervertebral disc degeneration. Arthritis Res. Ther. 2005, 7, R732–R745.
- Wang, X.; Wang, H.; Yang, H.; Li, J.; Cai, Q.; Shapiro, I.M.; Risbud, M.V. Tumor Necrosis Factor-α– and Interleukin-1β–Dependent Matrix Metalloproteinase-3 Expression in Nucleus Pulposus Cells Requires Cooperative Signaling via Syndecan 4 and Mitogen-Activated Protein Kinase–NF-κB Axis: Implications in Inflammatory Disc Disease. Am. J. Pathol. 2014, 184, 2560–2572.
This entry is offline, you can click here to edit this entry!
