Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Biochemistry & Molecular Biology
Phytohormone transporters, alkaloid transporters, ion and ion chelator transporters, sugar transporters, and amino acid transporters are common active transporters in plants. The examples of phytohormone transporters, alkaloid transporters, and ion and ion chelator transporters include ATP-binding cassette (ABC) transporters and Multidrug And Toxic compound Extrusion (MATE) transporters, while the examples of sugar transporters include MonoSaccharide Transporters (MSTs) and SUcrose Transporters (SUTs). These transporters have been reported to play important roles in various biological processes including cellular detoxification, nutrient transport, and stress adaptation.
- Sugar
- Transporters
- plants
- Stress responses
1. Classification and structural Properties of Sugar Transporters
In plants, MonoSaccharide Transporters (MSTs), SUcrose Transporters (SUTs; or SUCs, SUcrose Carriers), and Sugars Will Eventually be Exported Transporters (SWEETs) are the three major types of sugar transporters [1][2]. SUTs and MSTs belong to the major facilitator superfamily (MFS), which has the characteristic 12 transmembrane domains (TMDs), in the form of six N-terminal TMDs connected to six C-terminal TMDs via a cytosolic loop [1][3][4]. Despite having similar architectures, SUTs and MSTs could be differentiated from each other by the different domain structures [1]. On the other hand, SWEETs have seven TMDs and are characterized by an MtN3/saliva domain [1]. In terms of the transport mechanism, MSTs and SUTs are proton/sucrose symporters [5][6][7], while SWEETs are reported to be uniporters of sugars [5][6]. In the following sections, MSTs and SUTs, whose functions are H+-dependent, will be discussed.
2. The SUT Family
In photosynthetic cells, sucrose is derived from the photosynthetically fixed carbon and is the major form of sugar transported via the phloem to other tissues [8][9]. SUTs are H+/sucrose symporters involved in loading sucrose into the phloem against the concentration gradient and are driven by the proton motive force across the plasma membrane of the sieve element–companion cell complex (SE-CCC) [10].
2.1. The Activities of SUTs Are pH-Dependent
The pH dependence of SUT activities has been reported in various species. For example, in Arabidopsis, AtSUC4 was reported to be a proton/sucrose symporter localized in the vacuole membrane [11]. The proton motive force was suggested to be provided by the pumping of cytosolic protons into the vacuole by V-type ATPases and V-PPases [11]. Using yeast as the model, the proton-coupled and pH-dependent uptake of sucrose mediated by AtSUT4 was demonstrated [12]. Further experiments showed that AtSUC4 could act as a H+/sucrose antiporter or symporter depending on the pH difference between vacuole lumen and the medium outside [11]. When the vacuole lumen was more acidic than the medium outside, sucrose was imported into the vacuole with the export of H+ from the vacuole; when the medium outside the vacuole was more acidic, sucrose was transported together with proton into the vacuole [11]. Such switch of antiporter/symporter activity is illustrated in Figure 1. Similar bidirectional transport of sucrose was demonstrated using the phloem-localized ZmSUT1 [13]. The sucrose/H+ symporter activity of ZmSUT1 was demonstrated to be pH-dependent [13]. It was suggested that both the sucrose gradient and the proton motive force determine the sucrose/proton symport direction [13].
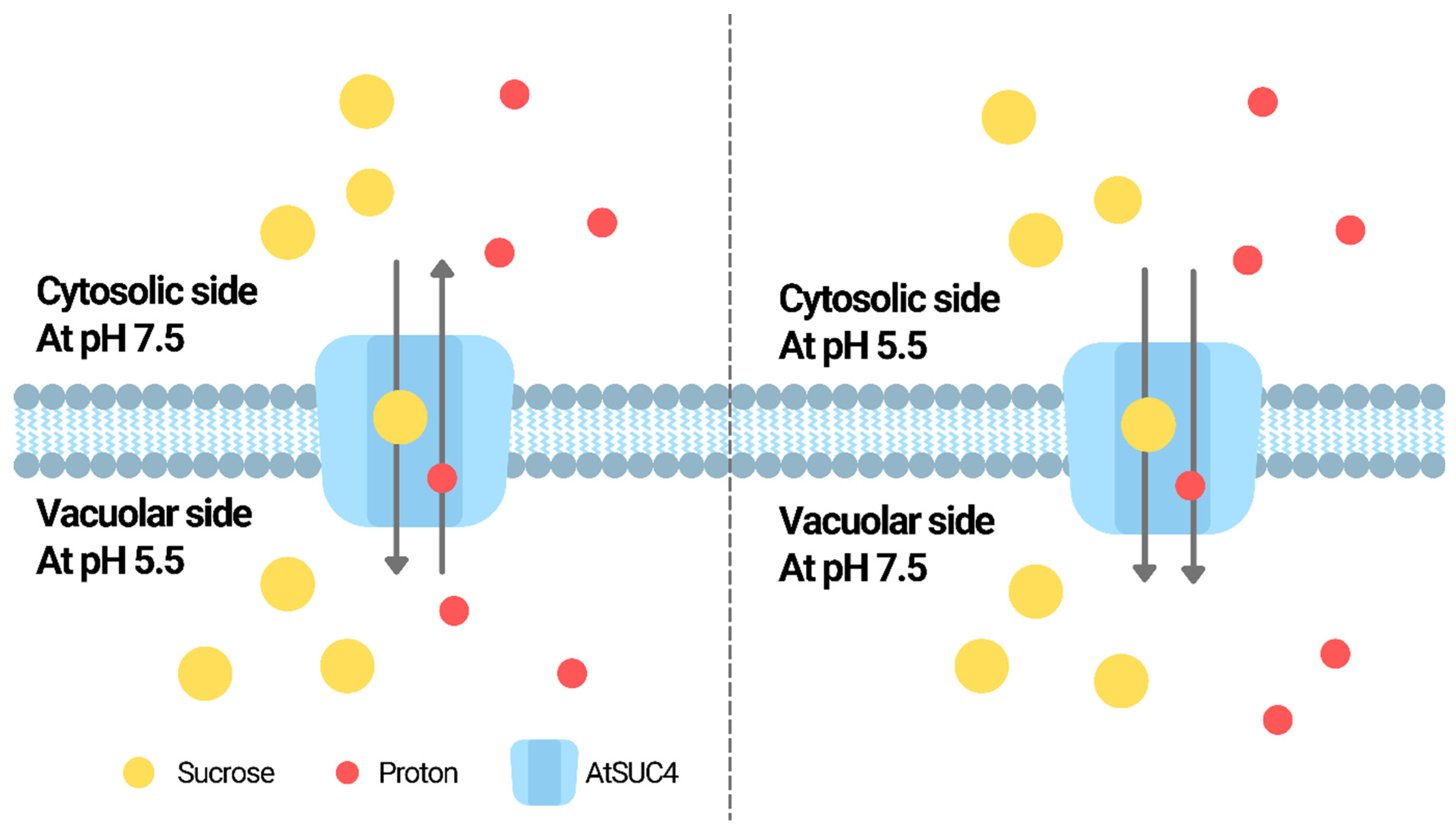
Figure 1. The switch of antiporter/symporter activity of AtSUC4. When the vacuole lumen was more acidic than the medium outside, AtSUC4 mediates the import of sucrose into the vacuole with the export of H+ from the vacuole; when the medium outside the vacuole was more acidic, AtSUC4 mediates the transport of sucrose together with proton into the vacuole [11].
2.2. The Role of Sucrose Transport during Stress-Induced Cellular pH Fluctuations
Atsuc4 mutants were found to be more sensitive to stresses including salt, osmotic, cold, and ABA treatments compared to the wild-type [14]. Probably due to the impaired sugar distribution, these mutants were found to have higher and lower sucrose, fructose and glucose in the shoots and roots, respectively, compared to the wild-type [14]. The ABA-induced expressions of stress-responsive genes, including ABA-responsive element binding factors (ABFs) and the genes upstream or downstream to ABFs, were inhibited in Atsuc4 mutants [14]. Although the detailed mechanism remained unclear, it was suggested that AtSUC4 is involved in the crosstalk between ABA signaling and sucrose signaling [14]. The accumulation of sucrose in the root is agriculturally important for those crops that are harvested for their edible roots. In sweet potato (Ipomoea batatas), similar to AtSUC4, IbSUT4 was found to regulate the accumulation of sucrose in the root and ABA signaling under stress [15]. The overexpression of IbSUT4 in Arabidopsis reduced the sucrose level in the leaf but improved the sucrose level in the root under salt stress [15]. This is possibly the result of a drop in cytosolic pH induced by salt stress [16], which reduced the sucrose export from the vacuole by SUT4.
The Phaseolus vulgaris SUT1.1, when expressed in transgenic yeast, was found to have a higher transport activity when the pH of the medium was more acidic [17]. The tonoplast- and plasma membrane-localized PvSUT1.1 was reported to be a sucrose-proton co-transporter that is probably involved in the export of sucrose from the leaf through the phloem [17]. In the same study, the expression of PvSUT1.1 was found to be repressed in the leaf upon heat stress [17]. Since the export of sucrose from the leaf is important for heat tolerance, the heat stress susceptibility of P. vulgaris was therefore hypothesized to be associated with the repression of PvSUT1.1 under high temperature [17]. However, under high temperature in the leaf, H+ can leak through membranes [18]. Although not discussed, such proton leaks in the cytosol may influence the transport activity of SUT in the tonoplast and plasma membrane. The net effect on the export of sucrose from the cell is unknown. To explain the SUT activity under stress, more mechanistic considerations, other than transcriptional controls, will be needed.
It has been widely accepted that SUTs are responsible for phloem loading. However, the analyses of expression data, including microarray, RNAseq, and quantitative PCR, from 167 experiments on various plant species showed that the effects on SUT expressions by factors such as photosynthetic rate, light level, and CO2 concentration are limited [19]. It was speculated that when the photosynthetic rate is increased, the existing SUT proteins may already have enough capacity to increase phloem loading without the need to increase their transcript levels [19]. Other regulatory mechanisms, such as differential intracellular localization and protein dimerization, were suggested [19]. Mechanistically, the changes in proton distribution under different environmental conditions could also potentially alter the SUT activity. Using Amaranthus caudatus and Vicia faba as the models for C4 and C3 plants, respectively, despite different kinetics and extents, the C4 and C3 plants both exhibited light-dependent cytosolic alkalization and vacuolar acidification [20]. Such alkalization and acidification peaked when the photosynthetic apparatus was maximally energized under high energy flux rates in the absence of CO2 [20]. Under such conditions, it is likely that the SUT activity will be affected. Therefore, the expression levels of SUTs under different environmental conditions could not sufficiently explain the regulation of the SUT function.
3. The MST Family
3.1. Classification and Structural Properties
Based on the protein sequences, MSTs can be further classified into several clades, including the STP, HXT, PLT-VGT, SBG-GLT-GLUT1, ERD6-like, and TMT clades [7]. Monosaccharides including glucose, fructose, galactose, mannose, and xylose are possible substrates of MSTs [21].
Based on the crystal structure of STP10 from A. thaliana that has recently been resolved [22][23], it was suggested that the protein’s affinity for sugar is mainly due to the N-terminal domain and the Lid domain of the protein, while the substrate specificity is mediated by the C-terminal domain, which interacts with specific hydroxyl groups of the substrate [22][23]. At the apoplast, which is acidic, protonation of the Asp42 residue occurs and finetunes the protein structure to enhance the binding affinity to the substrate [22]. The subsequent unloading of glucose then enables a modification in the protein structure, which results in the release of the proton from Asp42 [23]. Since the important functional domains for the proton-coupled substrate transport are conserved among STPs, it was suggested that this transport model explains the general mechanism of action of the transporter [22][23].
3.2. The Activities of MSTs Are pH-Dependent
In A. thaliana, AtPLT5 (polyol transporter 5) was reported to be a plasma membrane-localized MST-like protein that mediates the transport of sorbitol, glucose, galactose, ribose, xylose, mannitol, glycerol, and inositol [24]. Using transgenic Xenopus oocytes as the model and glucose and glycerol as substrate examples, the transport activity of AtPLT5 was demonstrated to be pH-dependent [24]. Maximal transport activity was demonstrated at pH 5.5 [24]. At pH 6.5, the transport activity was reduced; at pH 7.5, there was no transport activity [24]. When the extracellular pH was brought back to 5.5, the transport activity resumed [24]. AtPLT5 has a broad spectrum of substrates and is found to be expressed in various tissues [24]. Therefore, it is proposed that AtPLT5 is possibly involved in the retrieval of sugars from the apoplast [24]. The pH-dependent sugar/proton symporter activity of STPs was also reported in apple (Malus domestica) [25]. MdSTP13a was reported to be the transporter of both hexose and sucrose competitively to provide the sugars for pollen tube growth [25]. Using transgenic yeast as the model, optimal glucose or sucrose uptake by MdSTP13a was established at pH 6 [25]. An increase or decrease in the pH resulted in declined transport activity [25]. Different STPs have different optimal pH for their transport activities. For example, DgSTP1 from Datisca glomerata has the optimal pH for transport activity at pH 4.5 [26]. In plants, the transport of sugars is a major strategy to distribute or store nutrients [10]. Since different cellular compartments have different pH, understanding the pH dependence of the activities of sugar transporters is essential for the interpretation of the biological functions.
3.3. Paralogs of MSTs Have Differential Expression Patterns to Serve Different Functions
In A. thaliana, AtSTP1 was found to have a consistently high expression level in both the root and leaf among all 14 of the STPs identified, under normal conditions [27]. However, in the root, the expression of AtSTP13 was highly inducible by salinity and ABA treatment [27]. Although the expression of ATSTP1 in the root was also induced by salinity, the fold change is much less than that of AtSTP13 [27]. Both stp1 and stp13 mutants had reduced abilities to uptake glucose and fructose, while stp1 also had reduced galactose uptake [27]. After salt treatment, the leak of glucose from the stp13 mutant was enhanced [27]. Based on the expression data and the sugar flux data, it was suggested that AtSTP13 mediates the reabsorption of monosaccharides leaked from damaged cells under salt stress while AtSTP1 is the major contributor of monosaccharide uptake under normal conditions [27]. Such differential functions of STPs in the same species are in line with another expression study on STPs in O. sativa. The expressions of STPs were found to be responsive to stresses including cold, high temperature, and submergence [28]. However, the patterns of expression upon the same stress were diverse among various STPs, which also had different expression patterns in different tissues [28].
This entry is adapted from 10.3390/ijms23052824
References
- Misra, V.A.; Wafula, E.K.; Wang, Y.; DePamphilis, C.W.; Timko, M.P. Genome-wide identification of MST, SUT and SWEET family sugar transporters in root parasitic angiosperms and analysis of their expression during host parasitism. BMC Plant Biol. 2019, 19, 196.
- Doidy, J.; Vidal, U.; Lemoine, R. Sugar transporters in Fabaceae, featuring SUT MST and SWEET families of the model plant Medicago truncatula and the agricultural crop Pisum sativum. PLoS One 2019, 14, e0223173.
- Wang, Y.; Chen, Y.; Wei, Q.; Wan, H.; Sun, C. Phylogenetic relationships of sucrose transporters (SUTs) in plants and genome-wide characterization of SUT genes in Orchidaceae reveal roles in floral organ development. PeerJ 2021, 9, e11961.
- Drew, D.; North, R.A.; Nagarathinam, K.; Tanabe, M. Structures and general transport mechanisms by the major facilitator superfamily (MFS). Chem. Rev. 2021, 121, 5289–5335.
- Jeena, G.S.; Kumar, S.; Shukla, R.K. Structure, evolution and diverse physiological roles of SWEET sugar transporters in plants. Plant Mol. Biol. 2019, 100, 351–365.
- Eom, J.; Chen, L.; Sosso, D.; Julius, B.T.; Lin, I.W.; Qu, X.; Braun, D.M.; Frommer, W.B. SWEETs, transporters for intracellular and intercellular sugar translocation. Curr. Opin. Plant Biol. 2015, 25, 53–62.
- Lalonde, S.; Frommer, W.B. SUT sucrose and MST monosaccharide transporter inventory of the Selaginella genome. Front. Plant Sci. 2012, 3, 24.
- Lemoine, R.; Camera, S. La; Atanassova, R.; Dédaldéchamp, F.; Allario, T.; Pourtau, N.; Bonnemain, J.-L.; Laloi, M.; Coutos-Thévenot, P.; Maurousset, L.; et al. Source-to-sink transport of sugar and regulation by environmental factors. Front. Plant Sci. 2013, 4, 272.
- Nookaraju, A.; Upadhyaya, C.P.; Pandey, S.K.; Young, E.K.; Hong, J.S.; K, P.S.; Park, S.W. Molecular approaches for enhancing sweetness in fruits and vegetables. Sci. Hortic. (Amsterdam). 2010, 127, 1–15.
- Julius, B.T.; Leach, K.A.; Tran, T.M.; Mertz, R.A.; Braun, D.M. Sugar transporters in plants: new insights and discoveries. Plant Cell Physiol. 2017, 58, 1442–1460.
- Schulz, A.; Beyhl, D.; Marten, I.; Wormit, A.; Neuhaus, E.; Poschet, G.; Büttner, M.; Schneider, S.; Sauer, N.; Hedrich, R. Proton-driven sucrose symport and antiport are provided by the vacuolar transporters SUC4 and TMT1/2. Plant J. 2011, 68, 129–136.
- Weise, A.; Barker, L.; Kühn, C.; Lalonde, S.; Buschmann, H.; Frommer, W.B.; Ward, J.M. A new subfamily of sucrose transporters, SUT4, with low affinity/high capacity localized in enucleate sieve elements of plants. Plant Cell 2000, 12, 1345–1355.
- Carpaneto, A.; Geiger, D.; Bamberg, E.; Sauer, N.; Fromm, J.; Hedrich, R. Phloem-localized, proton-coupled sucrose carrier ZmSUT1 mediates sucrose efflux under the control of the sucrose gradient and the proton motive force. J. Biol. Chem. 2005, 280, 21437–21443.
- Gong, X.; Liu, M.; Zhang, L.; Ruan, Y.; Ding, R.; Ji, Y.; Zhang, N.; Zhang, S.; Farmer, J.; C, W. Arabidopsis AtSUC2 and AtSUC4, encoding sucrose transporters, are required for abiotic stress tolerance in an ABA-dependent pathway. Physiol. Plant. 2015, 153, 119–136.
- Wang, D.; Liu, H.; Wang, H.; Zhang, P.; Shi, C. A novel sucrose transporter gene IbSUT4 involves in plant growth and response to abiotic stress through the ABF-dependent ABA signaling pathway in Sweetpotato. BMC Plant Biol. 2020, 20, 157.
- Gao, D.; Knight, M.R.; Trewavas, A.J.; Sattelmacher, B.; Plieth, C. Self-reporting Arabidopsis expressing pH and [Ca2+] indicators unveil ion dynamics in the cytoplasm and in the apoplast under abiotic stress. Plant Physiol. 2004, 134, 898–908.
- Santiago, J.P.; Ward, J.M.; Sharkey, T.D. Phaseolus vulgaris SUT1.1 is a high affinity sucrose-proton co- transporter. Plant Direct 2020, 4, e00260
- Sharkey, T.D.; Zhang, R. High temperature effects on electron and proton circuits of photosynthesis. J. Integr. Plant Biol. 2010, 52, 712–722.
- Xu, Q.; Chen, S.; Yunjuan, R.; Chen, S.; Liesche, J. Regulation of sucrose transporters and phloem loading in response to environmental cues. Plant Physiol. 2018, 176, 930–945.
- Raghavendra, A.S.; Yin, Z.-H.; Heber, U. Light-dependent pH changes in leaves of C4 plants Comparison of the pH response to carbon dioxide and oxygen with that of C3 plants. Planta 1993, 189, 278–287.
- Slewinski, T.L. Diverse functional roles of monosaccharide transporters and their homologs in vascular plants: A physiological perspective. Mol. Plant 2011, 4, 641–662.
- Paulsen, P.A.; Custódio, T.F.; Pedersen, B.P. Crystal structure of the plant symporter STP10 illuminates sugar uptake mechanism in monosaccharide transporter superfamily. Nat. Commun. 2019, 10, 407.
- Bavnhøj, L.; Paulsen, P.A.; Flores-Canales, J.C.; Schiøtt, B.; Pedersen, B.P. Molecular mechanism of sugar transport in plants unveiled by structures of glucose/H+ symporter STP10. Nat. Plants 2021, 7, 1409–1419.
- Klepek, Y.; Geiger, D.; Stadler, R.; Klebl, F.; Landouar-arsivaud, L. Arabidopsis POLYOL TRANSPORTER5, a new member of the monosaccharide transporter-like superfamily, mediates H+-symport of numerous substrates, including myo-inositol, glycerol, and ribose. 2005, 17, 204–218.
- Li, C.; Meng, D.; Piñeros, M.A.; Mao, Y.; Dandekar, A.M.; Cheng, L. A sugar transporter takes up both hexose and sucrose for sorbitol-modulated in vitro pollen tube growth in apple. Plant Cell 2020, 32, 449–469.
- Schubert, M.; Koteyeva, N.K.; Wabnitz, P.W.; Santos, P.; Büttner, M.; Sauner, N.; Demchenko, K.; Pawlowski, K. Plasmodesmata distribution and sugar partitioning in nitrogen-fixing root nodules of Datisca glomerata. Planta 2011, 233, 139–152.
- Yamada, K.; Kanai, M.; Osakabe, Y.; Ohiraki, H.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Monosaccharide absorption activity of Arabidopsis roots depends on expression profiles of transporter genes under high salinity conditions. J. Biol. Chem. 2011, 286, 43577–43586.
- Kong, W.; An, B.; Zhang, Y.; Yang, J.; Li, S.; Sun, T.; Li, Y. Sugar transporter proteins (STPs) in Gramineae crops: comparative analysis, phylogeny, evolution, and expression profiling. Cells 2019, 8, 560.
This entry is offline, you can click here to edit this entry!
