Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Health Care Sciences & Services
In January 2021, the European Union ended the license of Mancozeb, the bestselling ethylenedithiocarbamate (EBDC) fungicide, because of some properties typical of human carcinogens. This decision contrasts the IARC classification of EBDC fungicides (Group 3, not classifiable as to human carcinogenicity).
- Zineb
- fungicides
- thyroid cancer
- melanoma
- brain cancer
- carcinogenesis
1. Introduction
The European Commission banned the use of Mancozeb, the bestselling ethylene-dithiocarbamate (EBDC) fungicide, from February 2021. Several regulatory agencies from Europe and the United States had previously examined EBDCs and their main metabolite, ethylene thiourea (ETU), with contradictory conclusive statements, based on the same available information. In 2001, the International Agency for Research on Cancer (IARC) Monograph N. 79 downgraded ETU from group 2B to Group 3 of human carcinogens (agent not classifiable as to its carcinogenicity to humans) [1]. Such a decision referred to the industrial uses of ETU as an accelerator for the vulcanization of polychloroprene and other rubbers, to its occurrence as an impurity in preparations of EBDC fungicides, and to its internal exposure as a product of EBDC metabolism. Such a decision underwent criticism [2] based on animal studies suggesting a goitrogenic effect and a link with thyroid cancer and a report showing increased TSH levels and genetic damage in agricultural workers exposed to EBDCs [3].In their reply against the criticism, two members of the IARC Monograph No. 79 Working Group motivated the decision with the formal evaluation procedure of the IARC Monographs and the lack of biological plausibility in transferring experimental animal results on thyroid cancer to humans [4]. However, while a joint consideration of the toxic effects of ETU and Mancozeb was unfeasible, the IARC supplement No.7, in re-evaluating ETU, contradicted that statement by extending the ETU Group 3 classification also to the EBDC fungicides Maneb, Thiram, Zineb, and Ziram, without presenting any further evidence [5,6,[5].
At that time, only one human study had evaluated the endocrine-disrupting and genotoxic effects of occupational exposure to EBDCs in agricultural settings, using urinary ETU excretion as a biomarker of exposure [3]. The Working Group motivated the decision of discarding it with its small size and the concurrent exposure to different EBDCs and organophosphates. Finally, a lack of evidence that ETU affected thyroid homeostasis in humans was asserted [4].
The EBDC fungicides, such as Mancozeb, Maneb, Zineb, Thiram, and Ziram, are widely used on a variety of agricultural and horticultural crops worldwide (Table 1). One dithiocarbamate, Disulfiram, is well known for its property to inhibit the enzyme alcohol dehydrogenase [6]. Such property is used in therapy to deter alcoholics from their dependence.
Table 1. Structural formula, year of marketing, physical status, and properties of the main ethylene-bis-dithiocarbamates (EBDC).
| Dithiocarbamates | Formula | Year Patented | Physical Status | Uses | IARC Last Evaluation (Year, Group) |
|---|---|---|---|---|---|
Disulfiram 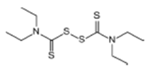 |
1900 | powder | Sulfur vulcanization of rubber; pharmaceutical treatment of alcoholism | 1976, 3 | |
Thiram 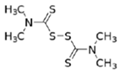 |
C6H12N2S4 | 1934 | powder | Accelerator in rubber vulcanization; pharmaceutical treatment of scabia; sun screen, bactericide; antifungal and animal repellant treatment of seeds, fruit, and ornamental shrubs | 1976, 3 |
Nabam 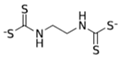 |
C4H6N2Na2S4 | 1943 | powder | Antifungal treatment of potato crops and various plants; biocide in sugar millas and pulp and paper mills | Not evaluated |
Ferbam 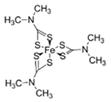 |
C9H18FeN3S6 | 1945 | Powder, wettable powder, liquid | Fungicide for fruit, nuts, vegetables, ornamental crops, and in household applications. | 1976, 3 |
Zineb 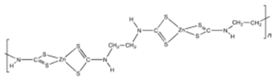 |
C4H6N2S4Zn | 1945 | Wettable powder | Antifungal treatment of seeds, vegetables, and various field and ornamental plants; additive in paints, fabrics, leather, linen, plastics, and wood surfaces | 1976, 3 |
Ziram 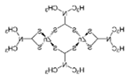 |
C6H12N2S4Zn | 1947 | Powder, wettable powder, liquid | Rubber accelerator; fungicide for fruit, vegetables, and ornamental crops. | 1976, 3 |
Maneb 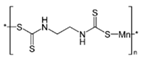 |
(C4H6MnN2S4)n | 1950 | Wettable powder | Fungicide for vegetables, seeds, nuts, field and forage crops, deciduous fruits, grapes, ornamental plants | 1976, 3 |
Metiram 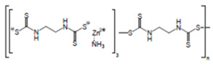 |
(C16H33N11S16Zn3)n | 1958 | Wettable powder | Fungicide for cereals, fruits, vegetables, tobacco, and ornamental plants. | Not evaluated |
Mancozeb 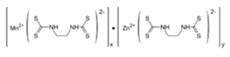 |
(C4H6MnN2S4)n | 1962 | Wettable powder | Fungicide for potato, vegetables, orchards, grapes, residential lawn, golf courses, athletic fields | Not evaluated |
Propineb 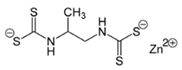 |
C5H8N2S4Zn | 1965 | Powder, wettable powder, liquid | Fungicide for fruit, grapes, tomatoes, potatoes, tobacco, rice, tea, and ornamental shrubs. | Not evaluated |
Dithiocarbamates were first synthesized from a monoamine and carbon disulphide, an inorganic solvent, to be used as accelerators in the rubber vulcanization. In 1934, tetramethylthiuram disulphide, known as Thiram, was the first dithiocarbamate to be patented as a fungicide, followed by more active compounds, such as Ferbam and Ziram, especially useful in herbal crops [7]. In 1943, a patent was awarded to the first EBDC, the Hester’s compound disodium ethylene bisdithiocarbamate (Nabam), which rapidly replaced copper sulphate in the treatment of many plant diseases and became popular among the potato growers in the United States, although its instability in the solid form complicated its practical handling. Shortly after Nabam introduction in the market, it was discovered that zinc sulphate had a stabilizing effect on the liquid. The reaction product between zinc sulphate and Nabam was zinc ethylene bisdithiocarbamate (Zineb). In 1950, DuPont replaced zinc with manganese and patented manganese ethylene bisdithiocarbamate (Maneb), which was more active than Nabam or Zineb. In 1962, Rohm and Haas registered the zinc ion complex of Maneb (Mancozeb), basically a combination of Maneb and Zineb, the bestseller among the EBDCs [6]. Currently, India accounts for 28% of the Mancozeb global market; 85% of sales are in Pacific Asia and Europe and only 4% in the United States. Further increase in its production and consumption is expected following the development of the Mancozeb production in China, up to an estimate of 250,000 metric tons in 2022 [7][8][9]. Potato and vegetable crops are the major uses (29 and 28% of sales, respectively), followed by orchards (19%) and vineyards (18%). The most important uses in the EU are against early and late blight in tomato and potato crops, the treatment of downy mildew on grapevines and vegetable crops, and the control of scab on pome fruit [7].
The 1996 Extension Toxicology Data Network (EXTOXNET) assessment of Mancozeb described it as practically nontoxic, causing mild skin and eye irritation and sensitization in rabbits and men. Concern was expressed for chronic exposure because of the occurrence of ETU as an impurity in Mancozeb formulations and its formation in the stored product, capable of causing goitre, birth defects, and cancer in experimental animals [10]. The U.S. Environmental Protection Agency re-evaluated Mancozeb in 2005 [11]. EPA acknowledged a hazard for occupational handlers of the fungicide due to the possibility of skin sensitization and allergy through all routes of administration and to endocrine-disrupting effects at the thyroid level resulting from the ETU metabolite. By extrapolating from the results in experimental animal studies, the cancer risk was defined as low [11]. A September 2008 document of the Swedish Chemicals Agency applied the European Council “cut off” criteria for placing plant protection products in the market to 271 approved active substances. Because of their endocrine disruptive effects, Maneb and Mancozeb were included among the 23 substances ineligible for approval [12]. The U.S. National Institutes of Health National Toxicology Program evaluated ETU in 2010: its conclusions highlighted the sufficient evidence of animal carcinogenicity, with the liver in mice and the thyroid gland in rats as the target organs and the occupational exposure to EBDCs as the main source of intake by inhalation, ingestion, and dermal contact [13]. In the United Kingdom, the Commission Working Group on the Classification and Labelling of Dangerous Substances took Mancozeb under consideration for the first time in November 1993, then again in 2003–2006. In 2017, the Chemicals Regulation Directorate of the U.K. Health and Safety Executive submitted to the European Chemical Agency (ECHA) a proposal for the classification and labelling of Mancozeb [14]. Mancozeb was considered a developmental toxicant. Its neurotoxicity was linked to the blockade of thyroid hormone synthesis, mediated by the inhibition of thyroid peroxidase by ETU, as the thyroid function is crucial for brain development in mammals. Mancozeb was also identified as a cause of allergic skin reaction described as not genotoxic and teratogenic at very high doses, capable of inducing maternal toxicity. Again, the reproductive toxicity at high-level exposure was attributed to ETU, which accounts for 7% Mancozeb metabolic conversion in the experimental animals. As it concerns its carcinogenicity, Mancozeb was defined as a medium-potency thyroid carcinogen in rats, with a non-genotoxic mechanism of action, which would cause thyroid tumours in humans only at implausibly high exposure levels. In absence of clear evidence linking hypothyroidism to thyroid cancer, the U.K. proposal was that Mancozeb classification for carcinogenicity was not required, consistent with the decision taken for ETU [14]. The ECHA opinion was adopted on 15 March 2019 [15]. Evidence that ETU altered thyroid function not only in rats but also in dogs and monkeys at relatively low doses prompted the conclusion that Mancozeb could induce thyroid toxicity in humans at dose levels relevant for classification in Category 2 for thyroid effects. The same was proposed for its neurotoxic effects. No mutagenic effects were detected. As for its carcinogenicity, the inadequacy of the epidemiological studies was highlighted, and the human relevance of the excess of follicular thyroid cancer observed among rats, but not mice, was discussed. The conclusion was that no classification was appropriate with the following arguments [15]:
-
Thyroid tumours in rats arise through inhibition of thyroid peroxidase (TPO) by ETU and/or Mancozeb leading to disruption of the HPT [hypothalamic-pituitary-thyroid] axis, a non-genotoxic mechanism of action.
-
ETU metabolism is more efficient in humans than in rats.
-
The plausible occurrence of the same mechanism of action in humans exhibits large, quantitative differences in respect to adult rats due to their lack of thyroxine-binding globulin (TBG).
-
Thyroid tumours are a relatively common finding in long-term rat studies, whilst the only known human thyroid carcinogen is ionizing radiation.
-
There is no clear evidence of an association between hypothyroidism and thyroid cancer in humans.
-
The epidemiological studies on EBDC exposure and thyroid cancer are negative.
-
A 1999 document by the European Chemicals Bureau (ECB) on thyroid tumours proposed that low- or medium-potency thyroid carcinogens in rodents should not be classified for human carcinogenicity.
-
Annex VI to the CLP Regulation for ETU, an agent causing thyroid tumours in rats and mice, does not classify ETU as a human carcinogen.
The same year, the European Food and Safety Authority reviewed the ecotoxicological implications of Mancozeb use [16]. The final document acknowledged the developmental toxicity and the endocrine disruption potential of the fungicide and the fact that occupational exposures and bystander exposures were above the acceptable operator exposure level (AOEL) in several crops, including potatoes, cereals, and grapevine. A high risk to birds and mammals from all uses was also highlighted [16]. As a result, on 14 December 2020, the EU State members voted to end the Mancozeb license in January 2021, therefore banning it in the EU from February 2021 [17].
2. Re-Evaluating the Human Carcinogenicity of Ethylenedithiocarbamate Fungicides
This analysis of the existing evidence of human carcinogenicity for EBDC fungicides and ETU highlights differences across molecules, multiple target organs, and research needs. A few studies suggest an increase in the risk of malignant melanoma and brain cancer among agricultural workers occupationally exposed to EBDC fungicides. In both instances, clinical and experimental evidence of the skin and the central nervous system as target organs for the EBDC toxicity lend credibility to these associations. While the results of the few available epidemiological studies on thyroid cancer are not suggestive for an association, the current evidence is based on: two ecological studies reporting contradictory results [18][19]; one small-size Russian cohort study on rubber workers presumably exposed to Thiram, reporting seven cases of thyroid enlargement and one case of thyroid cancer but no expected events [20]; one short, preliminary report of ETU-exposed workers with no detail whatsoever on exposure level, job title, length of exposure, or duration of follow-up and no indication about the expected events [21]; a third cohort study of researchers of a chemical plant where Mancozeb was also produced, with no reference to specific exposures [22]; and the U.S. AHS follow-up of pesticide use and incidence of thyroid cancer, with only five cases exposed to Mancozeb and/or Maneb [23]. No inference is possible from these studies, as the exposure was uncertain or indirectly assumed, or two or more EBDC were jointly considered, no attempts were made to explore trends by surrogates of exposure (such as duration), or the study size was too small. More research is warranted to explore the hypothesis with large, carefully designed epidemiological studies supported by a strong exposure assessment.
On the other hand, there is sufficient evidence supporting the carcinogenicity of most EBDC fungicides in experimental animals, with the thyroid as the main target in the rats and the liver in mice, with at least one study suggesting a multipotent carcinogenic effect on several other organs [24]. Notably, the EBDC and ETU thyrotoxicity observed in rats, dogs, and monkeys has also been shown in humans, and the link between thyroid disruption and cancer has been well demonstrated in human studies. Further studies are warranted to address the mechanism linking EBDC exposure to thyroid disrupting effects and thyroid cancer in men. It might be important to understand whether any difference in the health effects of specific EBDCs might be related to the release of manganese and zinc ions, consistent with what has been observed in relation to urinary levels of other metals [25][26]. Other mechanisms typical of some but not all EBDC fungicides, such as oxidative stress and genotoxicity, might also be relevant in human carcinogenesis.
Despite the existing evidence from experimental animal studies about their potential multisite carcinogenicity, the peculiar link between EBDC fungicides and ETU with thyroid cancer became the focus of some regulatory agencies, while the significance of thyroid tumours observed in rodents was considered of no relevance to humans and thus not warranting classification as carcinogenic [27]. Rats but not mice are more prone to develop thyroid tumours following chronic stimulation of the thyroid gland by high TSH levels. According to some authors, long-term assumption of drugs that enhance elimination of thyroid hormones would not affect the circulating T3, T4, and TSH levels in humans.
Such misinterpretation of findings, upon which the ECHA based its opinion on the classification and labelling of Mancozeb [14][15], and inaccurate accounts of the existing evidence [28] might have contributed to underestimating the toxicological and carcinogenic potential of EBDC fungicides. The current scientific evidence has refuted statements, such as “there is no clear evidence of an association between hypothyroidism and thyroid cancer in humans” [14][15] and “no nonradioactive chemical is known to cause [thyroid cancer] in humans” [27].
Twenty-five years later, it is time to reconsider the objections raised against the IARC decision to classify ETU as a Group 3 human carcinogen and to have separate judgments on this metabolite while evaluating it jointly with the parent substances. In fact, the ETU lack of genotoxic potential, whilst at least some of the parent EBDC fungicides do induce genetic damage, might suggest that other derivatives might be implicated. On the other hand, as ETU and EBDC fungicides share the thyroid disruptive potential, that would add to the opportunity of a joint re-evaluation.
3. Conclusions
To summarize, it might be profitable to use the IARC scheme as follows:
-
Human studies. The available evidence is inadequate to evaluate the human carcinogenicity of ethylenedithio-carbamates and ethylenethiourea, their main metabolite;
-
Animal studies. There is sufficient evidence of the carcinogenicity of ethylene-dithiocarbamates and ethylenethiourea in experimental animals;
-
Mechanistic evidence. There is sufficient evidence that the mechanisms responsible for the animal carcinogenicity of ethylene-dithiocarbamates and ethylenethiourea also apply to humans.
While the current scientific knowledge fully supports the EU decision to withdraw the license and to ban Mancozeb, it is time for the scientific community to focus on EBDC fungicides with new epidemiological studies supported by a state-of-the-art retrospective assessment of occupational exposure to EBDC fungicides and sufficient statistical power to detect the association. IARC should consider re-evaluating the potential human carcinogenicity of EBDC fungicides.
This entry is adapted from the peer-reviewed paper 10.3390/ijerph19052632
References
- International Agency for Research on Cancer. Some Thyrotropic Agents. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; IARC: Lyon, France, 2001; Volume 79, pp. 1–729.
- Steenland, K. Carcinogenicity of EBDCs. Environ. Health Perspect 2003, 111, A266.
- Steenland, K.; Cedillo, L.; Tucker, J.; Hines, C.; Sorensen, K.; Deddens, J.; Cruz, V. Thyroid hormones and cytogenetic outcomes in backpack sprayers using ethylenebis(dithiocarbamate) (EBDC) fungicides in Mexico. Environ. Health Perspect 1997, 105, 1126–1130.
- Baan, R.A.; Rice, J.M. Carcinogenicity of EBDC’s. Response. Environ. Health Perspect 2003, 111, A266–A267.
- International Agency for Research on Cancer. Overall Evaluation of Carcinogenicity: An updating of IARC Monographs Volumes 1 to 42. In IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Humans Supplement No. 7; IARC: Lyon, France, 1987; pp. 1–400.
- Vettorazzi, G.; Almeida, W.F.; Burin, G.J.; Jaeger, R.B.; Puga, F.R.; Rahde, A.F.; Reyes, F.G.; Schvartsman, S. International safety assessment of pesticides: Dithiocarbamate pesticides, ETU, and PTU—A review and update. Teratog. Carcinog. Mutagen. 1995, 15, 313–337.
- Cocco, P. Pesticides and Human health. Oxford Research Encyclopedias. In Environmental Health; Oxford University Press: New York, NY, USA, 2016.
- International Agency for Research on Cancer. Some Carbamates, Thiocarbamates, and Carbazides. In IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Humans; IARC: Lyon, France, 1976; Volume 12, pp. 137–150, 245–258.
- International Agency for Research on Cancer. Occupational exposures in pesticide applications and some pesticides. In IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Humans; IRAC: Lyon, France, 1991; Volume 53, pp. 1–586.
- Gullino, M.L.; Tinivella, F.; Garibaldi, A.; Kemmitt, G.M.; Bacci, L.; Sheppard, B. Mancozeb past, present, and future. Plant Dis. 2010, 94, 1076–1087.
- Industry Data Analytics. Mancozeb Market—Growth, Trends and Forecast (2021–2026) by types, by application, by regions and by key players: UPL, Coromandel International, Indofil, DowDuPont, 2020. Available online: https://www.industrydataanalytics.com/reports/global-Mancozeb-market (accessed on 1 December 2021).
- EXTOXNET Extension Toxicology Network. Pesticide Information Profiles. Mancozeb. Oregon State University. 1996. Available online: https://extoxnet.orst.edu/pips/Mancozeb.htm (accessed on 1 December 2021).
- U.S. Environmental Protection Agency. Prevention, Pesticides, and Toxic Substances. In Re-Registration Eligibility Decision for Mancozeb; EPA: Washington, DC, USA, 2005.
- Swedish Chemical Agency. Interpretation in Sweden of the Impact of the “Cut-Off” Criteria Adopted in the Common Position of the Council Concerning the Regulation of Placing Plant Protection Products on the Market (Document 11119/08). Available online: http://www.kemi.se/upload/Bekampningsmedel/Docs_eng/SE_positionpapper_annenII_sep08.pdf (accessed on 17 February 2022).
- U.S. National Institute of Environmental Health Sciences, National Toxicology Program. Report on Carcinogens. In Ethylene Thiourea, 14th ed.; U.S. Department of Health and Human Services: Washington, DC, USA, 2010. Available online: http://ntp.niehs.nih.gov/go/roc (accessed on 1 December 2021).
- U.K. Health and Safety Executive. Chemicals Regulation Directorate. Proposal for Harmonised Classification and Labelling. In CLH Report—Mancozeb; HSE: Bootle, UK, 2017.
- European Chemicals Agency. Committee for Risk Assessment—RAC. Opinion proposing harmonized classification and labelling at EU level of Mancozeb (ISO). In Manganese Ethylenebis (Dithiocarbamate) (Polymeric) Complex with Zinc Salt; ECHA: Helsinki, Finland, 2019; Available online: https://www.echa.europa.eu/documents/10162/6ea48bca-63ef-2999-1f1f-4ac1278d7b60 (accessed on 1 December 2021).
- Schreinemachers, D.M.; Creason, J.P.; Garry, V.F. Cancer Mortality in Agricultural Regions of Minnesota. Environ Health Perspect 1999, 107, 205–211.
- Nordby, K.-C.; Andersen, A.; Irgens, L.M.; Kristensen, P. Indicators of Mancozeb exposure in relation to thyroid cancer and neural tube defects in farmers’ families. Scand J. Work Environ. Health 2005, 31, 89–92.
- European Food Safety Authority; Abdourahime, H.; Anastassiadou, M.; Arena, M.; Auteri, D.; Barmaz, S.; Brancato, A.; Bura, L.; Carrasco Cabrera, L.; Chaideftou, E.; et al. Conclusion on the peer review of the pesticide risk assessment of the active substance Mancozeb. EFSA J. 2020, 18, 5755.
- The European Commission. Commission Implementing Regulation (EU) 2020/2087 of 14 December 2020 concerning the non-renewal of the approval of the active substance Mancozeb, in accordance with Regulation (EC) No 1107/2009 of the European Parliament and of the Council concerning the placing of plant protection products on the market and amending the Annex to Commission Implementing Regulation (EU) No 540/2011. 2020. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32020R2087&from=EN (accessed on 2 December 2021).
- Cherpak, V.V.; Bezuglyĭ, V.P.; Kaskevich, L.M. Health and hygienic characteristics of the working conditions and state of health of persons working with tetramethylthiuramdisulfide (TMTD). Vrach Delo 1971, 10, 136–139. (In Russian)
- Smith, D. Ethylene Thiourea—A study of possible teratogenicity and thyroid carcinogenicity. Occup. Med. 1976, 26, 92–94.
- Maher, K.V.; Defonso, L.R. A historical cohort study of mortality among chemical researchers. Arch. Environ. Health 1986, 41, 109–116.
- Lerro, C.C.; Beane Freeman, L.E.; Della Valle, C.T.; Andreotti, G.; Hofmann, J.N.; Koutros, S.; Parks, C.G.; Shrestha, S.; Alavanja, M.C.R.; Blair, A.; et al. Pesticide exposure and incident thyroid cancer among male pesticide applicators in agricultural health study. Environ. Int. 2021, 146, 106187.
- Belpoggi, F.; Soffritti, M.; Guarino, M.; Lambertini, L.; Cevolani, D.; Maltoni, C. Results of Long-Term Experimental Studies on the Carcinogenicity of Ethylene-bisdithiocarbamate (Mancozeb) in Rats. Ann. N. Y. Acad. Sci. 2002, 982, 123–136.
- Zhang, C.; Wu, H.-B.; Cheng, M.-X.; Wang, L.; Gao, C.-B.; Huang, F. Association of exposure to multiple metals with papillary thyroid cancer risk in China. Environ. Sci. Pollut. Res. Int. 2019, 26, 20560–20572.
- Zhang, Q.; Jiang, C.; Li, H.; Zhang, C.; Wu, H.; Huang, F. Effect of the Interaction Between Cadmium Exposure and CLOCK Gene Polymorphisms on Thyroid Cancer.; a Case-Control Study in China. Biol. Trace Elem. Res. 2020, 196, 86–95.
This entry is offline, you can click here to edit this entry!
