In the extraction of geniposide for the development of natural food colorants from the dried fruits of Gardenia jasminoides Rubiaceae, the gardenia fruit waste (GFW) still remaining 0.86% (w/w) of crocins has always been discarded without any further treatments. Crocins were extracted firstly by 50% of ethanol in the highest yield of 8.61 mg/g (w/w) from GFW. After the HPD-100 column fractionation in the collecting of crocins, the conversion ratio of 75% of crocins to crocetins can be obtained from the commercial available enzyme- Celluclast® 1.5 L. The crocins hydrolyzed products, were then separated through the HPD-100 resin adsorption and finally purified with the centrifugal partition chromatography (CPC) in single-step to obtain TC in a purity of 96.76 ± 0.17%. Conclusively, the effective enzyme transformation and purification co-operated with CPC technologies on crocins resulted in a high purity product of TC may be highly application in the commercial production.
- crocins
- trans-crocetin
- enzyme transformation
- centrifugal partition chromatography
1. The Percent Extractability and Total Content of Crocins in the Dried Gardenia Fruit
| Ethanol (%) | Yield of Extract (w/w %) 1 GF GFW | Total Content of Crocins (mg/g DW) 1 | ||
|---|---|---|---|---|
| GF | GFW | |||
| 25 | 19.90 ± 1.83 b | 10.28 ± 0.99 b | 10.28 ± 0.99 b | 4.15 ± 0.52 b |
| 50 | 25.63 ± 2.73 a | 14.09 ± 1.02 a | 14.09 ± 1.02 a | 8.61 ± 0.63 a |
| 75 | 14.26 ± 0.41 c | 9.20 ± 0.34 b | 9.20 ± 0.34 b | 6.13 ± 0.41 a |
| 95 | 10.77 ± 0.98 c | 4.09 ± 0.30 c | 4.09 ± 0.30 c | 1.33 ± 0.20 c |
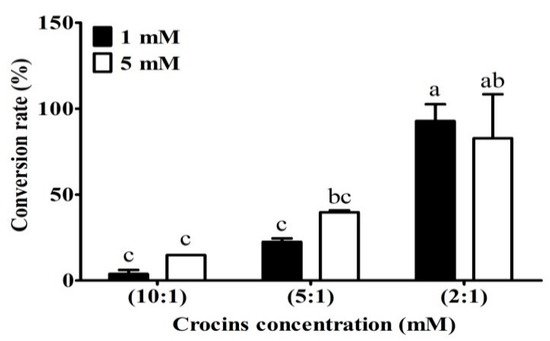
2. The Optimum Ratio of Enzyme to Crocins Required for the Conversion to Crocetin
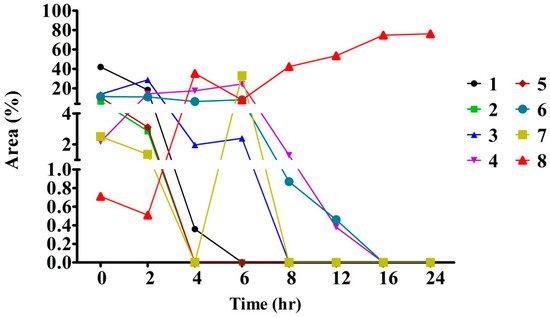
3. The Optimum Reaction Time Required for the Conversion of Crocins into Crocetin
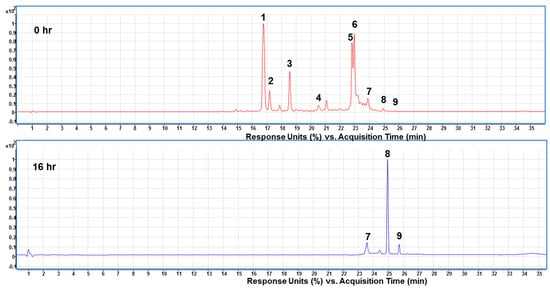
| Peak No. 1 | Retention Time (min) | λmax (nm) | Molecular Weight | Molecular Ion (m/z) | Fragmentation (m/z) 2 | Identified Crocins |
|---|---|---|---|---|---|---|
| 1 | 16.65 | 438, 466 | 976.96 | 999 [M + Na] + | 329, 311, 999 | trans-4-GG 3 |
| 2 | 17.13 | 440, 464 | 976.96 | 999 [M + Na] + | 635, 473, 999 | cis-4-GG 3 |
| 3 | 18.51 | 444, 464 | 814.82 | 837 [M + Na] + | 327, 837, 311 | trans-3-Gg 3 |
| 4 | 20.50 | 438, 460 | 652.26 | 675 [M + Na] + | 675, 323, 346 | trans-2-G 3 |
| 5 | 22.74 | 436, 460 | 976.96 | 999 [M + Na] + | 721, 311, 999 | cis-4-ng 3 |
| 6 | 22.91 | 442, 460 | 652.26 | 675 [M + Na] + | 675, 311, 329 | cis-2-G 3 |
| 7 | 23.87 | 430, 452 | 652.26 | 675 [M + Na] + | 675, 228, 329 | cis-2-gg 3 |
| 8 | 24.92 | 426, 450 | 328.40 | 329 [M + H] + | 329, 311, 293 | trans-Crocetin 4 |
| 9 | 25.70 | 424, 444 | 328.40 | 329 [M + H] + | 311, 329, 293 | cis-Crocetin 4 |
This entry is adapted from the peer-reviewed paper 10.3390/plants11030281
References
- Girme, A.; Pawar, S.; Ghule, C.; Shengule, S.; Saste, G.; Balasubramaniam, A.K.; Deshmukh, A.; Hingorani, L. Bioanalytical method development and validation study of neuroprotective extract of Kashmiri saffron using ultra-fast liquid chromatography-tandem mass spectrometry (UFLC-MS/MS): In Vivo pharmacokinetics of apocarotenoids and carotenoids. Molecules 2021, 26, 1815.
- Chemat, F.; Abert Vian, M.; Ravi, H.K.; Khadraoui, B.; Hilali, S.; Perino, S.; Fabiano Tixier, A.S. Review of alternative solvents for green extraction of food and natural products: Panorama, principles, applications and prospects. Molecules 2019, 24, 3007.
- Sakai, H.; Ono, K.; Tokunaga, S.; Sharmin, T.; Aida, T.M.; Mishima, K. Extraction of natural pigments from Gardenia jasminoides J. Ellis fruit pulp using CO2-expanded liquids and direct sonication. Separations 2021, 8, 1.
- Rosales-Calderon, O.; Trajano, H.L.; Duff, S.J. Stability of commercial glucanase and β-glucosidase preparations under hydrolysis conditions. Peer J. 2014, 2, e402.
- de Andrades, D.; Graebin, N.G.; Ayub, M.A.Z.; Fernandez-Lafuente, R.; Rodrigues, R.C. Preparation of immobilized/stabilized biocatalysts of β-glucosidases from different sources: Importance of the support active groups and the immobilization protocol. Biotechnol. Prog. 2019, 35, e2890.
- Suchareau, M.; Bordes, A.; Lemée, L. Improved quantification method of crocins in saffron extract using HPLC-DAD after qualification by HPLC-DAD-MS. Food Chem. 2021, 362, 130199.
- Bharate, S.S.; Kumar, V.; Singh, G.; Singh, A.; Gupta, M.; Singh, D.; Kumar, A.; Vishwakarma, R.A.; Bharate, S.B. Preclinical development of Crocus sativus-based botanical lead IIIM-141 for Alzheimer’s disease: Chemical standardization, efficacy, formulation development, pharmacokinetics, and safety pharmacology. ACS Omega 2018, 3, 9572–9585.
