Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Health Care Sciences & Services
In chronic degenerative diseases related to nitrogen metabolism and excretion, such as chronic kidney disease (CKD), the optimization of the amount of protein ingested in the diet is especially relevant. The occurrence of metabolic disorders and the nature of the amino acid mixture resulting from protein digestion, the general state of the patient and the severity of the CKD implies that proper protein intake should be carefully considered.
- CKD
- enteral nutrition
- protein ingestion
- mTOR
1. Introduction
In chronic degenerative diseases related to nitrogen metabolism and excretion, such as chronic kidney disease (CKD), the optimization of the amount of protein ingested in the diet is especially relevant. The occurrence of metabolic disorders and the nature of the amino acid mixture resulting from protein digestion, the general state of the patient and the severity of the CKD implies that proper protein intake should be carefully considered. CKD patients are usually classified into five stages according to eGFR and albuminuria, but for purposes of urea and other nitrogenous molecules, the five stages might be condensed into two large groups. Those in pre-dialysis with a conservative therapy, and those with advanced CKD that undergo periodical dialysis. In this context, the Kidney Disease Quality Initiative-National Kidney Foundation (KDOQI-NKF) guidelines for nutrition recommends a protein intake of 0.6 to 0.8 g/kg/day for pre-dialysis patients with CKD with an energy intake of 30 kcal/kg/day. On the other hand, the amount of protein recommended for advanced CKD patients is higher than 0.8 g/kg/day (up to 1.0–1.2) to compensate for the increased loss of protein and amino acids during dialysis [1,2]. The degree of albuminuria is also a key factor when adjusting the daily amount of protein.
Focusing on pre-dialysis CKD patients, the optimization in protein intake is necessary because of two conflicting factors. On the one hand, this disease requires a protein restriction in the diet to minimize general azotemia, which involves an excess of nitrogenous toxic molecules in the blood, mostly ammonium or urea. These metabolites originate as consequence of the protein tissue metabolism, including intestinal microbiota. On the other hand, a reduction in protein intake to minimize the contribution of the urea cycle could lead to a protein malnutrition risk with a consequent deficit in the supply of essential amino acids that are required for the replacement of body proteins [3]. This deficit would give rise to a loss in the protein synthesis capacity, which especially affects muscle tissue, producing lean muscle loss and sarcopenia [4,5], but also to other tissues, such as hepatic albumin synthesis. In that way, a deficit of protein ingestion puts these patients at a high risk of malnutrition [1].
Thus, it is assumed that one of the main goals for pre-dialysis CKD patients is the optimization of the amount and quality of protein intake for maintaining muscular proteostasis without the promotion of ureagenesis. However, the protein intake in CKD patients should account for a series of physiopathology factors usually related to CKD conditions. Aside from paying attention to the nitrogen balance between ingested and excreted nitrogen, the patients usually present altered metabolic situations, such as hormonal dysregulation, metabolic acidosis, insulin resistance, oxidative stress, chronic inflammation and hyperphosphatemia [6,7,8,9,10]. Thus, the increase of the oxidative stress levels is a prominent feature in CKD patients that must be considered.
2. CKD Side Effects and Their Usual Associated Conditions
Figure 1 displays the main metabolic disorders that can be associated with CKD patients. These patients show a significant decrease in the capacity of excretion of the nitrogenous toxic substance and hyperuremia. This is the main reason why a low protein-diet is recommended, and the preservation of muscular protein is compromised. In addition to that, clockwise, CKD patients used to have decreased levels of anabolic hormones but an increase in the secretion of several growth factors. These alterations increase the threshold of the nutrient sensing pathways such as the mTOR in the muscular tissue and simultaneously induce kidney hypertrophy and fibrosis damage in the glomerular and renal tissue, vasoconstriction, and other relevant effects.
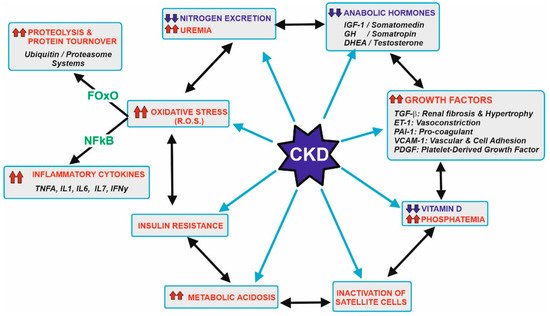
Figure 1. Metabolic abnormalities usually associated with patients with CKD. Starting at the left upper side, the gradual loss in kidney function leads to a decrease in the efficiency of nitrogen excretion and subsequent uremia. This should be related to recommend a control in the diet protein ingestion, which is the main goal of this review. Other abnormalities include a decrease in the levels of anabolic hormones, increase in a series of growth factors, decrease vitamin D and its effects, inactivation of muscle satellite cells needed for appropriate myocyte turnover, metabolic acidosis, and the development of insulin resistance, hyperglycemia and oxidative stress. Most of these effects are also interconnected by a complex network of signals. Oxidative stress induces transcription factors (majorly FOxO and NFkB) that increase damage in several tissues, including muscles due to higher proteolysis and the secretion of inflammatory cytokines. Further details are beyond the scope of this review, but appropriate references are cited at the text. Upward arrows in red indicate an increase, while downward arrows in blue indicate a decrease.
Other disturbances in these tissues include lowering the activation and beneficial action of vitamin D [20,21], inactivation of the differentiation of satellite cells needed for myocyte replacement [22], existence of metabolic acidosis [23], insulin resistance [24] and increase in oxidative stress [6,7,8,9,10]. The appearance of higher amounts of ROS and RNS (reactive oxygen and nitrogen species) induce the expression of two transcription factors, FoxO and NFκB [25,26]. These factors determine the presence of metabolic proteolysis and the secretion of a set of inflammatory cytokines that worsen the pathological conditions and increase the difficulties in maintaining muscular proteostasis. Vascular endothelium is also severely affected by oxidative stress. Endothelin-1 and other factors provoke vasoconstriction, while nitric oxide cannot counteract such effects. Conversely, the formation of peroxynitrites and other RNS/ROS causes significant damage, leading to a partial lost in the muscular vascularization [8].
3. Muscular Atrophy and Sarcopenia in CKD Patients
The tissue demanding the highest amount of amino acids for protein synthesis is the muscular tissue. In healthy subjects, approximately one third of the total daily amino acids are used by the muscles. Therefore, the muscular tissue is the most affected tissue in case of a low-protein diet recommended in pre-dialysis CKD patients. Muscular proteostasis is the result of an equilibrium between protein synthesis and protein degradation. Figure 2 shows the main factors and interactions that regulate both opposite processes in the myocyte.
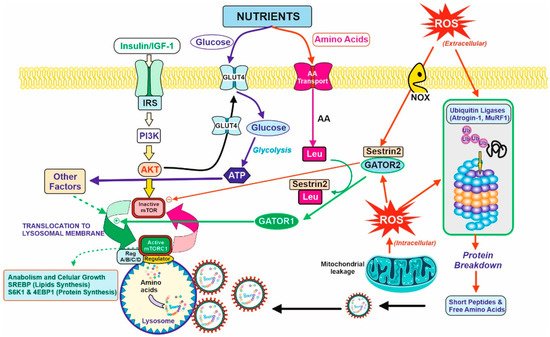
Figure 2. Balance between anabolic and catabolic signals in the myocyte. Nutrients and growth factors induce mTOR activation for protein synthesis. However, oxidative stress is caused by extracellular or intracellular ROS and accelerated protein degradation by the ubiquitin–proteasome system (by the induction of atrogenic genes. They are ubiquitin ligases (Atrogin-1 and MuRF1) that induce the formation of the protective protein Sestrin2 and diminish protein synthesis (right figure). Nutrients supply glucose for energy production (ATP) and amino acids for protein synthesis or catabolism, producing further energy and nitrogenous toxic compounds, mostly urea. Due to the last process, CKD patients should have limitations in terms of protein intake, and therefore, limits the bioavailability of amino acids. In this regard, Leu is partially important. Leu is needed for the activation of mTOR activity in the presence of Sestrin2 protein due to the oxidative stress situation. The binding of Leu to Sestrin2 promotes the action of GATOR1 for mTOR activation, although translocation to lysosomal membrane and other factors are required for complete activation and triggering anabolic processes (lipids and protein synthesis, left on the figure). CKD patients should have a counterbalance between the degradative and anabolic signals in the myocyte to avoid lean muscle loss with the minimal amount of protein to avoid excessive urea formation.
3.1. Protein Synthesis
Protein synthesis, as for other anabolic processes, is controlled and coordinated by the mTOR (mammalian Target Of Rapamycin) complex via a complex versatile and interconnected signal pathway network [27,28]. Briefly, two requirements are essential for mTOR activation; the first one concerns growth signals due to the binding of growth factors (mainly IGF-1, Insulin, but also GH) to its corresponding membrane receptor, and the second involves nutrients availability. Growth factors activate the PI3K/Akt signal pathway [29], one of the requirements for mTOR activation. In turn, this signal produces a translocation of GluT4 transporters to the membrane, increasing the transport of glucose inside the cell. With regard to amino acids, there are several transport mechanisms for the different families (not shown in Figure 2 for clarity). Although a well-balanced and proportional intracellular mixture is essential for protein synthesis, leucine is particularly important. Leucine acts as a trigger-sensor for the activation of the mTOR pathway because of its high affinity towards Sestrin2 [30]. Sestrins are stress-inducible metabolic proteins that protect organisms against various noxious stimuli, including DNA damage, oxidative stress, starvation, endoplasmic reticulum stress, and hypoxia. Furthermore, Sestrin-2 regulates the metabolism mainly by the activation of the key energy sensor AMP-dependent protein kinase (AMPK) and inhibition of the mammalian target of rapamycin complex 1 (mTORC1) [12]. Thus, Sestrin2 is usually linked to another protein, GATOR2, as a Sestrin2-GATOR2 complex that remains inactive the mTOR. The formation of the Leu–Sestrin2 complex promotes the release and subsequent conversion of GATOR2 to GATOR1, as an active effector of the mTOR pathway [31,32]. This displacement of the Sestrin2-GATOR2 so as to release GATOR1, and some other factors, allows for the translocation of mTOR to the periphery of the lysosomal membrane, and the full activation of the phosphorylation of key proteins for anabolic processes (mostly SREBP for lipid synthesis [33] and S6K1 plus 4E-BP1 for protein synthesis [34]).
3.2. Protein Degradation
Proteolysis of the myocyte proteins is stimulated by some transcription factors (FoxO and NFκB) related to oxidative stress, a usual condition in CKD as previously discussed (Figure 1). Extracellular and intracellular ROS generated by NOX membrane reactions or mitochondrial respiration induce: (i) the expression of atrogenic genes, mostly atrogin-1 and MuRF1 [35]. They act as ubiquitin ligases and increase the protein degradation rate via the ubiquitin-proteasome system [36,37], including (ii) the formation of Sestrin2, which is a member of the family of defense-proteins that protect cells against the ROS damage that is able to bind to GATOR2 and block protein synthesis by maintaining mTOR as an inactive form. In that way, oxidative stress accelerates protein degradation and inhibits protein synthesis, so that the equilibrium between both processes is broken, and the muscular proteostasis is lost. From an overall point of view, oxidative stress induces Sestrin2 expression, and this led to the necessity of higher levels of Leu for GATOR1 release and mTOR activation. In turn, Leu availability to the myocyte is diminished due to the partial loss of vascularization described in Section 2. Altogether, the anabolic threshold in the CKD patient is higher than in healthy people, as the higher levels of Sestrin2 plus the lower supply of Leu through the blood cannot trigger the same degree of mTOR activation.
This entry is adapted from the peer-reviewed paper 10.3390/nu14061182
This entry is offline, you can click here to edit this entry!
