Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Green & Sustainable Science & Technology
Cucurbita pepo, also referred to as “summer squash” or “zucchini”, originates from Central and South America, being currently cultivated worldwide in warm regions.
- Curcubita pepo seeds
- green extraction techniques
- nutraceutical industry
1. Introduction
C. pepo is an economically important crop in which immature fruits, leaves, peel, blossoms, and seeds are consumed due to their nutritional and medicinal benefits [1].
The seeds of the Cucurbitaceae family are globally used for the treatment of different diseases, particularly due to their antiviral, anti-inflammatory, anti-ulcerative, antidiabetic and antioxidant activities as well as analgesic for urinary disorders [2]. A few studies using C. pepo seed extracts were published in the past years, revealing wound healing [3], hair growth promotion [4] and anthelmintic [5] properties, besides their ability to inhibit the cell growth of hyperplastic and cancer cells [6]. Moreover, the treatment with C. pepo extract conducted to a substantial improvement of the lower urinary tract symptoms that are suggestive of benign prostatic hyperplasia [7].
According to different authors, C. pepo seeds are rich in several bioactive compounds, such as polyphenols [8], which have anti-aging and anticarcinogenic effects and may protect against vascular inflammation and cardiovascular and neurodegenerative diseases [9][10][11][12]. α- and γ-tocopherols [13] are also present, defending cells from oxidative stress and inflammation [14], while carotenoids (e.g., β-carotene and β-cryptoxanthin) may protect against different chronic diseases, including cancer [15] and cardiovascular diseases [16]. Besides that, carotenoids improve the cognitive and visual functions [17]. In addition, C. pepo seeds are rich in polyunsaturated fatty acids [18], zinc [19], and phytosterols such as β-sitosterol [13], which may reduce the blood cholesterol [20] and decrease the risk of certain types of cancer [21]. β-sitosterol is also the bioactive compound responsible for the successful use of C. pepo seeds in the treatment of benign prostatic hyperplasia [22][23][24]. Berberine and palmatine are also present in considerable amounts [5], conferring to nematocidal [5], antimalarial [25], antileishmaniasis [26], anti-schistosomiasis [27] and Toxoplasma gondii inhibitory properties [28].
Nevertheless, most of the authors employed organic solvents in the extractive step or, at least, used cold pressing methods aiming to obtain the lipidic fraction. Therefore, the use of water as an alternative solvent led to a greener extraction process and, simultaneously, avoided the extraction of a significant amount of lipids that are naturally present in C. pepo seeds. Allied to the selection of less-polluting solvents, it is imperative to select eco-friendly extraction methods. Therefore, technologies such as microwave-assisted extraction (MAE) and ultrasound-assisted extraction (UAE) arise as an alternative to the traditional ones [29]. MAE is a conventional, automated green extraction technique that allows the extraction of active components from different matrices [30][31]. By using microwave energy, MAE heats the solvents in contact with samples, disrupts the cell membrane, and releases the intracellular components into the solvents [31][32]. This is opposite to the conventional methods, such as Soxhlet extraction, that often requires 12–24 h of extraction periods and hundreds of milliliters of organic solvents [31]. When compared to such methods, MAE requires shorter extraction times, decreasing the degradation of the extracted components as well as the costs and the volume of the solvents used, and therefore improving the purity of the final extracts [33][34][35][36][37].
On the other hand, UAE is also an environmentally friendly, simple, effective, and inexpensive technique, with applications in pharmaceutical, cosmetic, and alimentary fields that have become more popular since 2007 [38][39][40]. This sustainable technique uses ultrasounds waves, which induce cavitation and thermal and mechanical effects in the extraction medium, and disrupt the cell walls of the matrix [39]. This phenomenon leads to the release of intracellular components into the solvents, without producing considerable modifications in the structure and properties of the compounds [41]. Some advantages of this technique include the use of small amounts of matrixes and solvents, short extraction times and samples throughput increment [42]. UAE also avoids the thermal decomposition of heat sensitive compounds, since it is a non-thermal process [43]. Furthermore, UAE achieves higher extraction yields in comparison with maceration and Soxhlet extractions [44]. Overall, these methodologies are less time-consuming, easy to execute, low-cost and usually lead to higher extraction yields, being reported by different authors as successfully in the recovery of high-added value compounds from plants [45][46][47].
Considering these points, the main goal of this study was the extraction of value-added compounds from C. pepo seeds using two green extraction methods, namely MAE and UAE, and water as solvent. Besides that, aiming to evaluate the influence of the ratio on the bioactive composition of the extracts, two different amounts of sample were used, namely 1 g/20 mL (condition 1) and 2.5 g/20 mL (condition 2). Afterwards, the extracts were characterized regarding antioxidant activity, radical scavenging capacity, phenolic profile, and in vitro cellular effects, aiming to determine their potential use as ingredients for nutraceutical purposes.
2. Antioxidant/Antiradical Activity
The TPC varied from 12.17 mg GAE/g DW (UAE condition 1) to 16.89 mg GAE/g DW (MAE condition 2). These results are in line with the results obtained by HPLC–PDA analysis (Section 3.4), which confirmed that the MAE condition 2 extract has the highest content in phenolic compounds, while the UAE condition 1 extract achieved one of the lowest. The TPC values are considerably higher than the ones obtained for C. pepo seeds extracted using acetone as a solvent in an Ultra Turax mixer (8.37 mg GAE/g DW) [48]. The TPC of MAE condition 2 extract is in line with the value reported by Mondal et al. [49] for an ethanolic extract of C. pepo leaves and stems (17.49 mg GAE/g DW), using the cold extraction method. Oppositely, conventional extracts of C. pepo fresh seeds dried at 65 °C and prepared with ultrapure water displayed a substantially lower content (2.39 mg GAE/g DW) [50]. The TPC values are also considerably higher than the ones reported for C. maxima seed oil extracted using chloroform/methanol (54.41 mg GAE/Kg) as solvent [51]. Moreover, Peiretti et al. obtained a TPC of 9.82 mg of catechin equivalents (CAE)/g of C. pepo seed extract using a mixture of 80:20 methanol/water (v/v) as solvent, and a solid material to solvent ratio of 1:10 (w/v) in an ultrasonic water bath [8]. Nevertheless, the units used by the authors are not the same, making it not possible to completely compare.
Regarding the DPPH assay, the results ranged from 4.35 mg TE/g DW (UAE condition 1) to 5.08 mg TE/g DW (MAE condition 2), with no significant differences between the extracts. These values are lower than the ones reported using conventional extracts of C. pepo fresh seeds dried at 65 °C and employing water as a solvent (118.19 μmol of TE/g) [50]. No more studies have been performed regarding the scavenging capacity of this radical.
In regards to the antioxidant activity evaluated by the FRAP assay, the MAE condition 1 extract achieved the best result (71.09 µmol FSE/g DW), while the UAE condition 2 extract achieved the worst (45.80 µmol FSE/g DW). These values are in line with the ones reported by Peirerri et al. (54 µmol FSE/g DW) for C. pepo seeds using 80:20 methanol/water (v/v) as a solvent [8]. Nevertheless, it should be highlighted that these authors used organic solvents, while in the present study, water was the only solvent employed.
Concerning the ABTS assay, the results varied between 11.38 mg AAE/g DW (UAE condition 2) and 13.29 mg AAE/g DW (MAE condition 1), with no significant differences between extracts. These results are not in accordance with the TPC ones, which could be justified by the ABTS mechanism of assay. The ABTS assay measures the relative ability of the antiradical compounds present in the extracts to scavenge the ABTS•+ cation generated in vitro. Nevertheless, some matrixes may have interferents, which do not scavenge the cation and lead to worse results. Therefore, the antiradical assays should always combine different radical assays (such as ABTS or DPPH).
Kulczyński et al. obtained an ABTS value of 79.30 mg Trolox/100 g DW for C. maxima seed extracts using an ultrasonic water bath for 1h at 30 °C, employing water as an extraction solvent [52]. Using ethanol and methanol as an extraction solvent, Nawirska–Olszańska et al. obtained ABTS values of 7.92 and 1.95 μM Trolox/g fresh weight (FW), respectively, using C. pepo (Miranda cultivar) seed extracts and the UAE technique [53]. Nevertheless, the results obtained in the present study could not be compared with these ones since the units are different.
3. Reactive Oxygen Species Scavenging Capacity Assays
Reactive species are the principal cause of oxidative stress, being divided in reactive oxygen and nitrogen species (ROS and RNS, respectively). These molecules, produced during the body aerobic metabolism, may cause oxidative damage of amino acids, DNA, lipids, and proteins [54][55][56].
Reactive oxygen species scavenging capacity (evaluated by O2●−, HOCl and ORAC assays) of C. pepo extracts prepared by microwave-assisted extraction and ultrasound-assisted extraction (MAE and UAE, respectively). Values are expressed as mean ± standard deviation (n = 3).
Among ROS, superoxide radical (O2●−) has particular importance as it is one of the most aggressive oxygen species in the human organism, being enrolled in the development of aging and chronic diseases, such as atherosclerosis, ischemic heart disease, diabetes mellitus, cancer, neurodegenerative diseases, immunosuppression and others [57][58][59][60]. Phenolic compounds have demonstrated a strong scavenging capacity of superoxide anion [61]. Concerning this species quenching assay, gallic acid and catechin were the best scavengers, with IC50 values of 24.55 µg/mL and 84.40 µg/mL, respectively, followed by MAE condition 2 (IC50 = 134.59 µg/mL), UAE condition 2 (IC50 = 178.68 µg/mL) and UAE condition 1 (IC50 = 221.88 µg/mL). It is important to emphasize that there are no significant differences (p > 0.05) between MAE condition 2 extract and catechin used as positive control.
Among the ROS studied, the best results were achieved by hypochlorous acid (HOCl). For this species, the UAE condition 1 (IC50 = 1.88 µg/mL) and MAE condition 1 (IC50 = 2.29 µg/mL) extracts achieved the best results, being not significantly different from gallic acid (IC50 = 3.27 µg/mL) used as positive control. These results are followed by MAE condition 2 (IC50 = 6.32 µg/mL) and UAE condition 2 (IC50 = 13.50 µg/mL) extracts.
Regarding the oxygen radical absorbance capacity, the values of C. pepo extracts varied between 0.04 µg TE/mg DW (MAE condition 2) and 1.13 µg TE/mg DW (UAE condition 1), with no significant difference between the extracts and the positive controls employed.
To the best of knowledge, this is the first study that screened the radical scavenging activity of C. pepo extracts. Nevertheless, comparing with other seed matrixes, the results are very interesting. For example, using tea seed oil extracted by cold pressing, Liu et al. obtained a IC50 = 1.73 mg/mL for the O2●− radical [62], which indicates that this extract has a lower radical scavenging capacity than the C. pepo extracts tested. Annatto seed extracts, obtained by a conventional extraction employing distilled water as the solvent, displayed an IC50 = 1.0 µg/mL for the HOCl and an IC50 = 0.11 µmol TE/mL for the ORAC assay. However, no O2●− radical scavenging activity was detected within the assayed concentration range (15–25 µg/mL) [63].
4. Phenolic Compounds of C. pepo Seed Extract
Phenolic compounds are a group of non-enzymatic antioxidants that have the ability to combat free radicals, which, when in excess, may lead to increased oxidative stress and consequently to cell aging.
MAE condition 2 achieved the highest concentration of phenolic compounds (20.34 mg/g DW), followed by MAE condition 1 (17.01 mg/g DW), UAE condition 1 (12.51 mg/g DW) and UAE condition 2 (9.15 mg/g DW). The lower concentration of phenolic compounds in the UAE extracts may be explained by the partial degradation of phenolic acids by ultrasonic waves and the creation of highly reactive hydroxyl radicals during the UAE process, which has been reported by several authors [64][65][66].
In all extracts, the phenolic compound present in the highest quantity was catechin. Quantitatively, in addition to catechin, the compounds identified in higher amounts were caffeine and gallic acid in the UAE condition 1 and UAE condition 2 extracts, and caffeine, phloridzin, gallic acid, protocatechuic acid, and chlorogenic acid in the MAE condition 1 and MAE condition 2 extracts. Moreover, caffeine, catechin, naringin, gallic acid, protocatechuic acid, caftaric acid, chlorogenic acid, 4-O-caffeyolquinic acid, vanillic acid, caffeic acid, syringic acid, p-coumaric acid, ferulic acid and 4,5-di-O-caffeoylquinic acid are present in all samples. Phloridzin and rutin were only detected in the UAE extracts. The phenolic compounds 3,5-di-caffeoylquinic acid, quercetin-3-O-galactoside, resveratrol, quercetin-3-O-glucopyranoside, ellagic acid, cinnamic acid, quercitrin, kaempferol-3-O-glucoside, isorhamnetin-3-O-glucoside, kaempferol-3-O-rutinoside, isorhamnetin-3-O-rutinoside, naringenin, trans-epsilon viniferin, quercetin, phloretin, tiliroside, kaempferol, apigenin and chrysin were not detected in any of the C. pepo extracts.
To the best of the knowledge, this is the first work that identified and quantified the phenolic compounds present in C. pepo seed extracts. Nevertheless, it is possible to compare the results with other Cucurbita seed extracts. The results obtained by Ennebs et al. shown that C. moschata seed extract contains less phenolic compounds than C. pepo, as only six phenolic acids (quinic acid, protocatechuic acid, caffeic acid, syringic acid, trans-ferulic acid and 4,5-Di-O-caffeoylquinic acid) were identified in C. moschata seed extracts using LC-ESI-MS, employing hexane, chloroform, ethyl acetate and methanol as the extraction solvent [67].
Employing ultrasounds as the extraction method and methanol/H2O 80:20 (v/v) as the solvent, Iswaldi et al. identified 10 phenolic acids (p-coumaric acid, ferulic acid, caftaric acid, caffeic acid, 3-O-caffeoylquinic acid, caffeic acid, 2-O-caffeoylmalic acid, dicaffeoyltartaric acid, dicaffeic acid and sinapic acid) and 16 flavonoids (including luteolin O-glucoside, quercetin 3-O-rhamnosyl-rhamnosyl-glucoside, quercetin rutinoside, glucoside, isorhamnetin 3-rutinoside-7-rhamnoside and different kaempferol glycosides) in C. pepo whole fruit extracts, using LC-DAD-Q-TOF MS [68].
5. Cytotoxic Effects of C. pepo Seed Extracts towards Intestinal and Neuronal Cells
Caco-2, an intestinal cell line, was used to evaluate the cytotoxicity of the C. pepo extracts obtained by UAE and MAE. This cell line allows the study the compound absorption across the intestinal epithelium, being commonly employed to evaluate the extracts safety [69]. According to Figure 1, the exposure of this cell line to increasing concentrations (0.1–1000 µg/mL) of MAE C. pepo extracts showed a viability around 100%, without significant differences (p > 0.05). On the other hand, only the concentration of 0.1 μg/mL of the UAE condition 1 extract maintained a viability of 101.36%, being significantly different from the other concentrations (p < 0.05). At higher concentrations, the viability ranged between 70.20% (1000 μg/mL) and 77.82% (1 μg/mL). The UAE condition 2 displayed viabilities above 88.78%, being not significantly different (p > 0.05). The lower viability displayed by Caco-2 cells incubated with UAE C. pepo extracts may be explained by the higher content on neochlorogenic acid and caftaric acid present in the extracts. These compounds have antiproliferative activity and may effectively inhibit the growth of colorectal cancer cells [70][71].
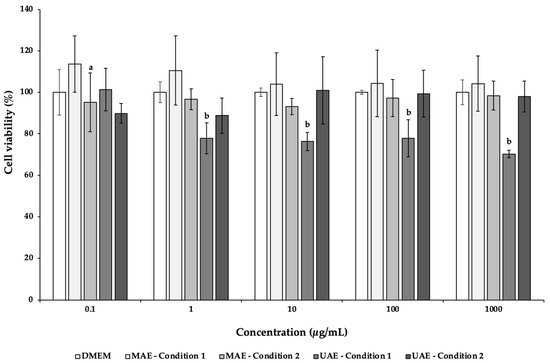
Figure 1. Effects of C. pepo extracts obtained by UAE and MAE exposure on the viability of Caco-2 cells at different concentrations (0.1–1000 µg/mL), measured by MTT assay. Values are expressed as mean ± standard deviation (n = 3). Different letters represent significant differences between concentrations of the same sample (p < 0.05), according to Tukey’s HSD test.
In the present study, NSC-34 cell line was used to evaluate the potential neurotoxicity of C. pepo extracts obtained by UAE and MAE (Figure 2). NSC-34 is a hybrid cell line that has similar morphological and physiological properties to motor neurons [72]. This cell line in culture mimics the synthesis and storage of acetylcholine (ACh) and the expression of neurofilament proteins [72]. After 24 h of incubation in the presence of different concentrations (0.1–1000 µg/mL) of C. pepo extracts, it was possible to observe a decrease of the NSC-34 cell viability, mainly in the MAE and UAE condition 1 extracts (Figure 2). Indeed, at the highest concentration tested (1000 µg/mL), the cells’ viability was 9.92% and 19.58%, for MAE condition 1 and UAE condition 1, respectively, with significant differences (p < 0.05). In these cases, it was possible to determine the IC50 values of 253.68 (MAE condition 1) and 95.32 µg/mL (UAE condition 1).
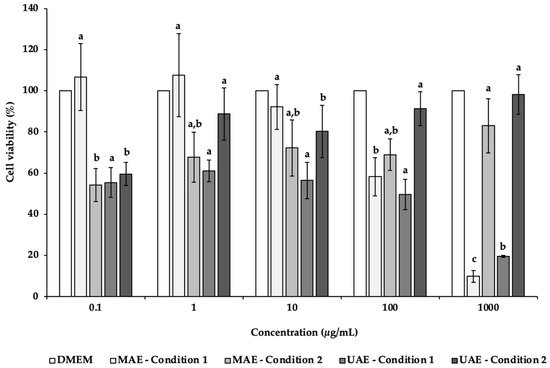
Figure 2. Effects of C. pepo extracts obtained by UAE and MAE exposure on the viability of NSC-34 cells at different concentrations (0.1–1000 µg/mL), as measured by the MTT assay. Values are expressed as mean ± standard deviation (n = 3). Different letters indicate significant differences between concentrations of the same sample (p < 0.05), according to Tukey’s HSD test.
Oppositely, MAE and UAE condition 2 showed an increasing trend with increased concentrations. For MAE condition 2, the results varied from 54.17% (0.1 μg/mL) to 82.97% (1000 μg/mL). In the case of UAE condition 2 extracts, the highest cell viability (98.21%) was found for the highest concentration tested (1000 μg/mL), following in descending order 100, 1, 10 and 0.1 μg/mL.
The inferior cells’ viability observed after exposure to MAE condition 1 and UAE condition 1 extracts may be due to the presence in higher amounts of the antiproliferative compounds neochlorogenic acid and caftaric acid, which is not observed in MAE condition 2 and UAE condition 2 extracts. Besides that, MAE condition 2 and UAE condition 2 extracts present other compounds (e.g., catechin, gallic acid or protocatechuic acid) with benefic cell effects in higher quantities, justifying a possible protective effect. Further studies are needed to justify these differences.
This entry is adapted from the peer-reviewed paper 10.3390/app12031763
References
- Kulczyński, B.; Sidor, A.; Gramza-Michałowska, A. Antioxidant Potential of Phytochemicals in Pumpkin Varieties Belonging to Cucurbita Moschata and Cucurbita Pepo Species. CyTA-J. Food 2020, 18, 472–484.
- Gutierrez, R. Review of Cucurbita Pepo (Pumpkin) its Phytochemistry and Pharmacology. Med. Chem. 2016, 6, 12–21.
- Rabrenović, B.B.; Dimić, E.B.; Novaković, M.M.; Tešević, V.V.; Basić, Z.N. The most Important Bioactive Components of Cold Pressed Oil from Different Pumpkin (Cucurbita Pepo L.) Seeds. LWT—Food Sci. Technol. 2014, 55, 521–527.
- Hajhashemi, V.; Rajabi, P.; Mardani, M. Beneficial Effects of Pumpkin Seed Oil as a Topical Hair Growth Promoting Agent in a Mice Model. Avicenna J. Phytomed 2019, 9, 499–504.
- Grzybek, M.; Kukula-Koch, W.; Strachecka, A.; Jaworska, A.; Phiri, A.M.; Paleolog, J.; Tomczuk, K. Evaluation of Anthelmintic Activity and Composition of Pumpkin (Cucurbita Pepo L.) Seed Extracts—in vitro and in vivo Studies. Int. J. Mol. Sci. 2016, 17, 1456.
- Medjakovic, S.; Hobiger, S.; Ardjomand-Woelkart, K.; Bucar, F.; Jungbauer, A. Pumpkin Seed Extract: Cell Growth Inhibition of Hyperplastic and Cancer Cells, Independent of Steroid Hormone Receptors. Fitoterapia 2016, 110, 150–156.
- Vahlensieck, W.; Theurer, C.; Pfitzer, E.; Patz, B.; Banik, N.; Engelmann, U. Effects of Pumpkin Seed in Men with Lower Urinary Tract Symptoms due to Benign Prostatic Hyperplasia in the One-Year, Randomized, Placebo-Controlled GRANU Study. Urol. Int. 2014, 94, 286–295.
- Peiretti, P.G.; Meineri, G.; Gai, F.; Longato, E.; Amarowicz, R. Antioxidative Activities and Phenolic Compounds of Pumpkin (Cucurbita Pepo) Seeds and Amaranth (Amaranthus Caudatus) Grain Extracts. Nat. Prod. Res. 2017, 31, 2178–2182.
- Piccolella, S.; Crescente, G.; Candela, L.; Pacifico, S. Nutraceutical Polyphenols: New Analytical Challenges and Opportunities. J. Pharm. Biomed. Anal. 2019, 175, 112774.
- Serino, A.; Salazar, G. Protective Role of Polyphenols against Vascular Inflammation, Aging and Cardiovascular Disease. Nutrients 2018, 11, 53.
- Brglez Mojzer, E.; Knez Hrnčič, M.; Škerget, M.; Knez, Ž.; Bren, U. Polyphenols: Extraction Methods, Antioxidative Action, Bioavailability and Anticarcinogenic Effects. Molecules 2016, 21, 901.
- Losada-Barreiro, S.; Bravo-Díaz, C. Free Radicals and Polyphenols: The Redox Chemistry of Neurodegenerative Diseases. Eur. J. Med. Chem. 2017, 133, 379–402.
- Kim, M.Y.; Kim, E.J.; Kim, Y.-N.; Choi, C.; Lee, B.-H. Comparison of the Chemical Compositions and Nutritive Values of Various Pumpkin (Cucurbitaceae) Species and Parts. Nutr. Res. Pr. 2012, 6, 21–27.
- Wallert, M.; Ziegler, M.; Wang, X.; Maluenda, A.; Xu, X.; Yap, M.L.; Witt, R.; Giles, C.; Kluge, S.; Hortmann, M.; et al. α-Tocopherol Preserves Cardiac Function by Reducing Oxidative Stress and Inflammation in Ischemia/Reperfusion Injury. Redox Biol. 2019, 26, 101292.
- Jian, L.; Du, C.J.; Lee, A.H.; Binns, C.W. Do Dietary Lycopene and other Carotenoids Protect against Prostate Cancer? Int. J. Cancer 2005, 113, 1010–1014.
- Sesso, H.D. Carotenoids and Cardiovascular Disease: What Research Gaps Remain? Curr. Opin. Lipidol. 2006, 17, 11–16.
- Moran, N.E.; Mohn, E.S.; Hason, N.; Erdman, J.W., Jr.; Johnson, E.J. Intrinsic and Extrinsic Factors Impacting Absorption, Metabolism, and Health Effects of Dietary Carotenoids. Adv. Nutr. 2018, 9, 465–492.
- Ryan, E.; Galvin, K.; O’Connor, T.P.; Maguire, A.; O’Brien, N.M. Phytosterol, Squalene, Tocopherol Content and Fatty Acid Profile of Selected Seeds, Grains, and Legumes. Mater. Veg. 2007, 62, 85–91.
- Glew, R.; Glew, R.; Chuang, L.-T.; Huang, Y.-S.; Millson, M.; Constans, D.; VanderJagt, D. Amino Acid, Mineral and Fatty Acid Content of Pumpkin Seeds (Cucurbita Spp.) and Cyperus Esculentus Nuts in the Republic of Niger. Mater. Veg. 2006, 61, 49–54.
- Piironen, V.; Lindsay, D.; Miettinen, T.; Toivo, J.; Lampi, A.-M. Plant Sterols: Biosynthesis, Biological Function and their Importance to Human Nutrition. J. Sci. Food Agric. 2000, 80, 939–966.
- Raicht, R.F.; Cohen, B.I.; Fazzini, E.P.; Sarwal, A.N.; Takahashi, M. Protective Effect of Plant Sterols against Chemically Induced Colon Tumors in Rats. Cancer Res. 1980, 40, 403–405.
- Gossell-Williams, M.; Davis, A.; O’connor, N. Inhibition of Testosterone-Induced Hyperplasia of the Prostate of Sprague-Dawley Rats by Pumpkin Seed Oil. J. Med. Food 2006, 9, 284–286.
- Zerafatjou, N.; Amirzargar, M.; Biglarkhani, M.; Shobeirian, F.; Zoghi, G. Pumpkin Seed Oil (Cucurbita Pepo) Versus Tamsulosin for Benign Prostatic Hyperplasia Symptom Relief: A Single-Blind Randomized Clinical Trial. BMC Urol. 2021, 21, 1–7.
- Carbin, B.-E.; Larsson, B.; Lindahl, O. Treatment of Benign Prostatic Hyperplasia with Phytosterols. Br. J. Urol. 1990, 66, 639–641.
- Leitao da-Cunha, E.V.; Fechine, I.M.; Guedes, D.N.; Barbosa-Filho, J.M.; Sobral da Silva, M. Protoberberine alkaloids. In The Alkaloids: Chemistry and Biology; Cordell, G.A., Ed.; Academic Press: Cambridge, MA, USA, 2005; Volume 62, pp. 1–75.
- Vennerstrom, J.L.; Lovelace, J.K.; Waits, V.B.; Hanson, W.L.; Klayman, D.L. Berberine Derivatives as Antileishmanial Drugs. Antimicrob. Agents Chemother. 1990, 34, 918–921.
- Dkhil, M. Role of Berberine in Ameliorating Schistosoma Mansoni-Induced Hepatic Injury in Mice. Biol. Res. 2014, 47, 1–7.
- Krivogorsky, B.; Pernat, J.A.; Douglas, K.A.; Czerniecki, N.J.; Grundt, P. Structure–Activity Studies of Some Berberine Analogs as Inhibitors of Toxoplasma Gondii. Bioorg. Med. Chem. Lett. 2012, 22, 2980–2982.
- Panja, P. Green Extraction Methods of Food Polyphenols from Vegetable Materials. Curr. Opin. Food Sci. 2018, 23, 173–182.
- Kataoka, H. New Trends in Sample Preparation for Analysis of Plant-Derived Medicines. Curr. Org. Chem. 2010, 14, 1698–1713.
- Llompart, M.; Garcia-Jares, C.; Celeiro, M.; Dagnac, T. Microwave-Assisted Extraction. In Encyclopedia of Analytical Science, 3rd ed.; Worsfold, P., Poole, C., Townshend, A., Miró, M., Eds.; Academic Press: Oxford, UK, 2019; pp. 67–77.
- Kataoka, H. Pharmaceutical Analysis | sample preparation. In Encyclopedia of Analytical Science, 3rd ed.; Worsfold, P., Poole, C., Townshend, A., Miró, M., Eds.; Academic Press: Oxford, UK, 2019; pp. 231–255.
- Ahuja, S.; Diehl, D. Chapter 2 Sampling and Sample Preparation. In Comprehensive Analytical Chemistry; Elsevier: Amsterdam, The Netherlands, 2006; Volume 47, pp. 15–40.
- Sasaki, K.; Honda, W.; Ohsawa, S.; Miyake, Y.; Kawashima, Y. A Study of Microwave Sterilizer for Injection Ampules (no.4): Application to Sterilization of Thermally Labile Drug Solutions. J. Pharm. Sci. Technol. Jpn. 1998, 58, 125–135.
- Beejmohun, V.; Fliniaux, O.; Grand, É.; Lamblin, F.; Bensaddek, L.; Christen, P.; Kovensky, J.; Fliniaux, M.-A.; Mesnard, F. Microwave-Assisted Extraction of the Main Phenolic Compounds in Flaxseed. Phytochem. Anal. 2007, 18, 275–282.
- Proestos, C.; Komaitis, M. Application of Microwave-Assisted Extraction to the Fast Extraction of Plant Phenolic Compounds. LWT 2008, 41, 652–659.
- Gallo, M.; Ferracane, R.; Graziani, G.; Ritieni, A.; Fogliano, V. Microwave Assisted Extraction of Phenolic Compounds from Four Different Spices. Molecules 2010, 15, 6365–6374.
- Rostagno, M.A.; Prado, J.M. Natural Product Extraction: Principles and Applications; RSC Green Chemistry: Cambridge, UK, 2013.
- Reddy, A.V.B.; Moniruzzaman, M.; Madhavi, V.; Jaafar, J. Recent Improvements in the Extraction, Cleanup and Quantification of Bioactive Flavonoids; Elsevier: Amsterdam, The Netherlands, 2020; Volume 66, pp. 197–223.
- Roohinejad, S.; Nikmaram, N.; Brahim, M.; Koubaa, M.; Khelfa, A.; Greiner, R. Chapter 16-potential of novel technologies for aqueous extraction of plant bioactives. In Water Extraction of Bioactive Compounds; Dominguez González, H., González Muñoz, M.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 399–419.
- Moldoveanu, S.; David, V. Chapter 6-solvent extraction. In Modern Sample Preparation for Chromatography, 2nd ed.; Moldoveanu, S., David, V., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 191–279.
- Ishtiaq, F.; Farooq, R.; Farooq, U.; Farooq, A.; Siddique, M.; Shah, S.H.; Shaheen, M. Application of Ultrasound in Pharmaceutics. World Appl. Sci. J. 2009, 6, 886–893.
- Ma, Y.-Q.; Ye, X.-Q.; Fang, Z.-X.; Chen, J.-C.; Xu, G.-H.; Liu, D.-H. Phenolic Compounds and Antioxidant Activity of Extracts from Ultrasonic Treatment of Satsuma Mandarin (Citrus Unshiu Marc.) Peels. J. Agric. Food Chem. 2008, 56, 5682–5690.
- Sun, Y.; Liu, Z.; Wang, J. Ultrasound-Assisted Extraction of Five Isoflavones from Iris Tectorum Maxim. Sep. Purif. Technol. 2011, 78, 49–54.
- Moreira, M.M.; Barroso, M.F.; Boeykens, A.; Withouck, H.; Morais, S.; Delerue-Matos, C. Valorization of Apple Tree Wood Residues by Polyphenols Extraction: Comparison between Conventional and Microwave-Assisted Extraction. Ind. Crop. Prod. 2017, 104, 210–220.
- Pimentel-Moral, S.; Borrás-Linares, I.; Lozano-Sánchez, J.; Arráez-Román, D.; Martínez-Férez, A.; Segura-Carretero, A. Microwave-Assisted Extraction for Hibiscus Sabdariffa Bioactive Compounds. J. Pharm. Biomed. Anal. 2018, 156, 313–322.
- Chemat, F.; Rombaut, N.; Sicaire, A.-G.; Meullemiestre, A.; Fabiano-Tixier, A.-S.; Abert-Vian, M. Ultrasound Assisted Extraction of Food and Natural Products. Mechanisms, Techniques, Combinations, Protocols and Applications. A Review. Ultrason. Sonochem. 2017, 34, 540–560.
- Ethiraj, S.; Balasundaram, J. Phytochemical and Biological Activity of Cucurbita Seed Extract. J. Adv. Biotechnol. 2016, 6, 813–821.
- Mondal, S.; Hossain, I.; Islam, M. Determination of Antioxidant Potential of Cucurbita Pepo Linn. (An Edible Herbs of Bangladesh). J. Pharmacogn. Phytochem. 2017, 6, 1016–1019.
- Saavedra, M.J.; Aires, A.; Dias, C.; Almeida, J.A.; De Vasconcelos, M.C.B.M.; Santos, P.; Rosa, E.A. Evaluation of the Potential of Squash Pumpkin by-Products (Seeds and Shell) as Sources of Antioxidant and Bioactive Compounds. J. Food Sci. Technol. 2013, 52, 1008–1015.
- Rezig, L.; Chouaibi, M.; Ojeda-Amador, R.M.; Gomez-Alonso, S.; Salvador, M.D.; Fregapane, G.; Hamdi, S. Cucurbita Maxima Pumpkin Seed Oil: From the Chemical Properties to the Different Extracting Techniques. Not. Bot. Horti Agrobot. Cluj-Napoca 2018, 46, 663–669.
- Kulczyński, B.; Gramza-Michałowska, A.; Królczyk, J.B. Optimization of Extraction Conditions for the Antioxidant Potential of Different Pumpkin Varieties (Cucurbita Maxima). Sustainability 2020, 12, 1305.
- Nawirska-Olszańska, A.; Kita, A.; Biesiada, A.; Sokół-Łętowska, A.; Kucharska, A.Z. Characteristics of Antioxidant Activity and Composition of Pumpkin Seed Oils in 12 Cultivars. Food Chem. 2013, 139, 155–161.
- Gutteridge, J.M. Lipid Peroxidation and Antioxidants as Biomarkers of Tissue Damage. Clin. Chem. 1995, 41, 1819–1828.
- Halliwell, B. Antioxidant Characterization: Methodology and Mechanism. Biochem. Pharmacol. 1995, 49, 1341–1348.
- Kehrer, J.P. Free Radicals as Mediators of Tissue Injury and Disease. Crit. Rev. Toxicol. 1993, 23, 21–48.
- Sánchez-Moreno, C.; Larrauri, J.A.; Saura-Calixto, F. Free Radical Scavenging Capacity and Inhibition of Lipid Oxidation of Wines, Grape Juices and Related Polyphenolic Constituents. Food Res. Int. 1999, 32, 407–412.
- Malencić, D.; Gasic, O.; Popović, M.; Boza, P. Screening for Antioxidant Properties of Salvia Reflexa Hornem. Phytother Res. 2000, 14, 546–548.
- Gülçin, I.; Oktay, M.; Küfrevioğlu, O.I.; Aslan, A. Determination of Antioxidant Activity of Lichen Cetraria Islandica (L) Ach. J. Ethnopharmacol. 2001, 79, 325–329.
- Devasagayam, T.P.A.; Tilak, J.C.; Boloor, K.K.; Sane, K.S.; Ghaskadbi, S.S.; Lele, R.D. Free Radicals and Antioxidants in Human Health: Current Status and Future Prospects. J. Assoc. Physicians India 2004, 52, 794–804.
- Shahidi, F.; Janitha, P.K.; Wanasundara, P.D. Phenolic Antioxidants. Crit. Rev. Food Sci. Nutr. 1992, 32, 67–103.
- Liu, G.; Zhu, W.; Zhang, J.; Song, D.; Zhuang, L.; Ma, Q.; Yang, X.; Liu, X.; Zhang, J.; Zhang, H.; et al. Antioxidant Capacity of Phenolic Compounds Separated from Tea Seed Oil in Vitro and in Vivo. Food Chem. 2021, 371, 131122.
- Chisté, R.C.; Mercadante, A.Z.; Gomes, A.; Fernandes, E.; Lima, J.; Bragagnolo, N. In Vitro Scavenging Capacity of Annatto Seed Extracts against Reactive Oxygen and Nitrogen Species. Food Chem. 2011, 127, 419–426.
- Karabegović, I.T.; Stojičević, S.S.; Veličković, D.T.; Todorovic, Z.; Nikolić, N.; Lazić, M.L. The Effect of Different Extraction Techniques on the Composition and Antioxidant Activity of Cherry Laurel (Prunus Laurocerasus) Leaf and Fruit Extracts. Ind. Crop. Prod. 2014, 54, 142–148.
- Bubalo, M.C.; Ćurko, N.; Tomašević, M.; Ganić, K.K.; Redovniković, I.R. Green Extraction of Grape Skin Phenolics by Using Deep Eutectic Solvents. Food Chem. 2016, 200, 159–166.
- Sharmila, G.; Nikitha, V.; Ilaiyarasi, S.; Dhivya, K.; Rajasekar, V.; Kumar, N.; Muthukumaran, K. Ultrasound Assisted Extraction of Total Phenolics from Cassia Auriculata Leaves and Evaluation of its Antioxidant Activities. Ind. Crop. Prod. 2016, 84, 13–21.
- Enneb, S.; Drine, S.; Bagues, M.; Triki, T.; Boussora, F.; Guasmi, F.; Nagaz, K.; Ferchichi, A. Phytochemical Profiles and Nutritional Composition of Squash (Cucurbita Moschata D.) from Tunisia. S. Afr. J. Bot. 2020, 130, 165–171.
- Iswaldi, I.; Gómez-Caravaca, A.M.; Lozano-Sánchez, J.; Arraez-Roman, D.; Carretero, A.S.; Gutierrez, A.F. Profiling of Phenolic and other Polar Compounds in Zucchini (Cucurbita Pepo L.) by Reverse-Phase High-Performance Liquid Chromatography Coupled to Quadrupole Time-of-Flight Mass Spectrometry. Food Res. Int. 2013, 50, 77–84.
- Artursson, P.; Karlsson, J. Correlation between Oral Drug Absorption in Humans and Apparent Drug Permeability Coefficients in Human Intestinal Epithelial (Caco-2) Cells. Biochem. Biophys. Res. Commun. 1991, 175, 880–885.
- Szwajgier, D.; Paduch, R.; Kukuła-Koch, W.; Polak-Berecka, M.; Waśko, A. Study on Biological Activity of Bread Enriched with Natural Polyphenols in Terms of Growth Inhibition of Tumor Intestine Cells. J. Med. Food 2020, 23, 181–190.
- Elansary, H.O.; Mahmoud, E.A. In Vitro Antioxidant and Antiproliferative Activities of Six International Basil Cultivars. Nat. Prod. Res. 2015, 22, 2149–2154.
- Cashman, N.R.; Durham, H.D.; Blusztajn, J.K.; Oda, K.; Tabira, T.; Shaw, I.T.; Dahrouge, S.; Antel, J.P. Neuroblastoma × Spinal Cord (NSC) Hybrid Cell Lines Resemble Developing Motor Neurons. Dev. Dyn. 1992, 194, 209–221.
This entry is offline, you can click here to edit this entry!
