The cornea is an avascular connective tissue that is crucial, not only as the primary barrier of the eye but also as a proper transparent refractive structure. Corneal transparency is necessary for vision and is the result of several factors, including its highly organized structure, the physiology of its few cellular components, the absence of blood and lymphatic vessels in healthy conditions, the tightly controlled hydration state, and the lack of myelinated nerves, among others. The cornea is supplied by both sensory and autonomic nerves, being one of the most densely innervated tissues in the body. Corneal innervation is anatomically organized into four levels ranging from the nerve trunks in the corneal stroma to the nerve terminals in the epithelium. Electrophysiological recordings of corneal sensory nerve fibers have revealed the existence of three different functional types of sensory neurons that are classified into mechanonociceptors, polymodal nociceptors and cold thermoreceptors depending on the modality of stimuli by which they are activated. The impulse discharge is conducted by these neurons to the central nervous system, where sensory input is processed to finally evoke a sensation and to regulate ocular protective functions, such as tearing and blinking.
- cornea
- corneal anatomy
- corneal innervation
- corneal nerves
1. Introduction
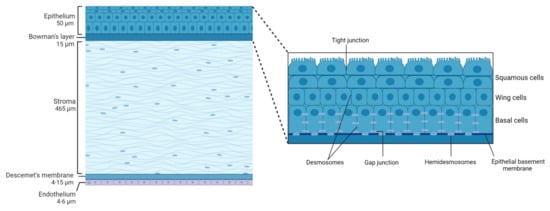
2. Corneal Structure
2.1. Epithelium
2.2. Bowman’s Layer
2.3. Stroma
2.4. Descemet’s Membrane
2.5. Endothelium
3. Corneal Innervation
3.1. Corneal Nerve Architecture
Stromal Nerves
Subbasal Nerve Plexus
Intraepithelial Nerve Terminals
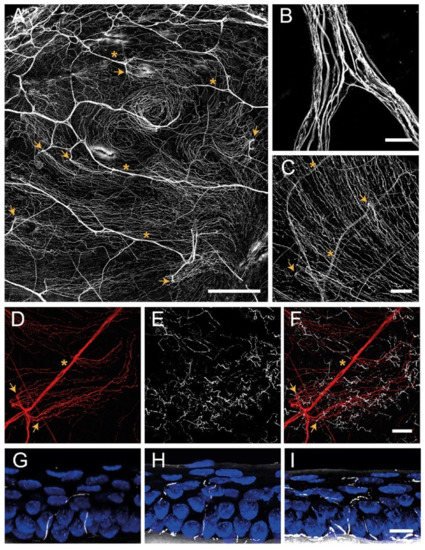
3.2. Functional Types of Corneal Nerves
Mechanonociceptors
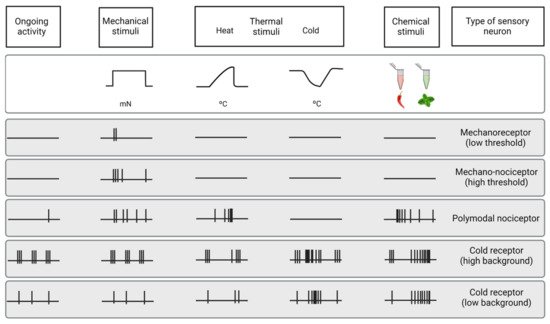
Polymodal Nociceptors
Cold Thermoreceptors
3.3 Changes of Nerve Activity under Inflammation and after Injury
This entry is adapted from the peer-reviewed paper 10.3390/ijms23062997
References
- Acosta, M.C.; Peral, A.; Luna, C.; Pintor, J.; Belmonte, C.; Gallar, J.; Tear secretion induced by selective stimulation of corneal and conjunctival sensory nerve fibers.. Investig. Ophthalmol. Vis. Sci. 2004, 45, 2333–2336, .
- Parra, A.; Madrid, R.; Echevarria, D.; del Olmo, S.; Morenilla-Palao, C.; Acosta, M.C.; Gallar, J.; Dhaka, A.; Viana, F.; Belmonte, C.; et al. Ocular surface wetness is regulated by TRPM8-dependent cold thermoreceptors of the cornea. . Nat. Med. 2010, 16, 1396–1399, .
- Quallo, T.; Vastani, N.; Horridge, E.; Gentry, C.; Parra, A.; Moss, S.; Viana, F.; Belmonte, C.; Andersson, D.A.; Bevan, S.; et al. TRPM8 is a neuronal osmosensor that regulates eye blinking in mice. . Nat. Commun. 2015, 6, 7150, .
- Alcalde, I.; Íñigo-Portugués, A.; González-González, O.; Almaraz, L.; Artime, E.; Morenilla-Palao, C.; Gallar, J.; Viana, F.; Merayo-Lloves, J.; Belmonte, C.; et al. Morphological and functional changes in TRPM8-expressing corneal cold thermoreceptor neurons during aging and their impact on tearing in mice.. J. Comp. Neurol. 2018, 526, 1859–1874, .
- Gallar, J.; Acosta, M.C.; Moilanen, J.A.O.; Holopainen, J.M.; Belmonte, C.; Tervo, T.M.T.; Recovery of corneal sensitivity to mechanical and chemical stimulation after laser in situ keratomileusis. . J. Refract. Surg. 2004, 20, 229–235, .
- Luna, C.; Quirce, S.; Aracil-Marco, A.; Belmonte, C.; Gallar, J.; Acosta, M.C.; Unilateral Corneal Insult Also Alters Sensory Nerve Activity in the Contralateral Eye.. Front. Med. 2021, 8, 2079, .
- Acosta, M.C.; Luna, C.; Quirce, S.; Belmonte, C.; Gallar, J.; Corneal sensory nerve activity in an experimental model of UV keratitis. . Investig. Ophthalmol. Vis. Sci. 2014, 55, 3403–3412, .
- Kovács, I.; Luna, C.; Quirce, S.; Mizerska, K.; Callejo, G.; Riestra, A.; Fernández-Sánchez, L.; Meseguer, V.M.; Cuenca, N.; Merayo-Lloves, J.; et al. Abnormal activity of corneal cold thermoreceptors underlies the unpleasant sensations in dry eye disease. . Pain 2016, 157, 399–417, .
- Belmonte, C.; Pain, Dryness, and Itch Sensations in Eye Surface Disorders Are Defined by a Balance between Inflammation and Sensory Nerve Injury.. Cornea 2019, 38, S11–S24, .
- Luna, C.; Mizerska, K.; Quirce, S.; Belmonte, C.; Gallar, J.; del Carmen Acosta, M.; Meseguer, V.; Sodium Channel Blockers Modulate Abnormal Activity of Regenerating Nociceptive Corneal Nerves After Surgical Lesion. . Investig. Opthalmol. Vis. Sci. 2021, 62, 2, .
- Gonzalez, G.G.; De la Rubia, P.G.; Gallar, J.; Belmonte, C.; Reduction of capsaicin-induced ocular pain and neurogenic inflammation by calcium antagonists. Investig. Ophthalmol. Vis. Sci. 1993, 34, 3329–3335, .
- Vitar, R.M.L.; Barbariga, M.; Fonteyne, P.; Bignami, F.; Rama, P.; Ferrari, G.; Modulating ocular surface pain through neurokinin-1 receptor blockade.. Investig. Ophthalmol. Vis. Sci. 2021, 62, 26, .
- Murata, Y.; Masuko, S.; Peripheral and central distribution of TRPV1, substance P and CGRP of rat corneal neurons. . Brain Res. 2006, 1085, 87–94, .
- Yang, L.; Mehta, J.; Liu, Y.C.; Corneal neuromediator profiles following laser refractive surgery. . Neural Regen. Res. 2021, 16, 2177–2183, .
- Zhang, X.; Mak, S.; Li, L.; Parra, A.; Denlinger, B.; Belmonte, C.; McNaughton, P.A.; Direct inhibition of the cold-Activated TRPM8 ion channel by Gα q.. Nat. Cell Biol. 2012, 14, 850–858, .
- Hanna, C.; Bicknell, D.S.; O’brien, J.E. Cell Turnover in the Adult Human Eye. Arch. Ophthalmol. 1961, 65, 695–698.
- Thoft, R.A.; Friend, J. The X, Y, Z hypothesis of corneal epithelial maintenance. Investig. Ophthalmol. Vis. Sci. 1983, 24, 1442–1443.
- Wilson, S.E. Bowman’s layer in the cornea– structure and function and regeneration. Exp. Eye Res. 2020, 195, 108033.
- Wilson, S.E.; Hong, J.W. Bowman’s layer structure and function: Critical or dispensable to corneal function? A Hypothesis. Cornea 2000, 19, 417–420.
- Lee, T. The ins and outs of corneal wound healing. Rev. Optom. 2016, 153, 44–56.
- Hazlett, L.D. Corneal and Ocular Surface Histochemistry. Prog. Histochem. Cytochem. 1993, 25, 3–6.
- Maurice, D.M. The transparency of the corneal stroma. Vision Res. 1970, 10, 107–108.
- Watsky, M.A. Keratocyte gap junctional communication in normal and wounded rabbit corneas and human corneas. Investig. Ophthalmol. Vis. Sci. 1995, 36, 2568–2576.
- Stramer, B.M.; Zieske, J.D.; Jung, J.C.; Austin, J.S.; Fini, M.E. Molecular mechanisms controlling the fibrotic repair phenotype in cornea: Implications for surgical outcomes. Investig. Ophthalmol. Vis. Sci. 2003, 44, 4237–4246.
- Haustein, J. On the ultrastructure of the developing and adult mouse corneal stroma. Anat. Embryol. 1983, 168, 291–305.
- Dua, H.S.; Faraj, L.A.; Said, D.G.; Gray, T.; Lowe, J. Human corneal anatomy redefined: A novel pre-Descemet’s layer (Dua’s layer). Ophthalmology 2013, 120, 1778–1785.
- Smith, R.S.; Sundberg, J.P.; John., S.W.M. The anterior segment and ocular adnexae. In Systematic Evaluation of the Mouse Eye: Anatomy, Pathology and Biomethods; Smith, R.S., Ed.; CRC Press: Boca Raton, FL, USA, 2002; Volume 1, pp. 3–23.
- Al-Aqaba, M.A.; Dhillon, V.K.; Mohammed, I.; Said, D.G.; Dua, H.S. Corneal nerves in health and disease. Prog. Retin. Eye Res. 2019, 73, 100762.
- Belmonte, C.; Tervo, T.T.; Gallar, J. Sensory innervation of the eye. In Adler’s Physiology of the Eye, 11th ed.; Elsevier: Amsterdam, The Netherlands, 2011; Volume 16, pp. 363–384.
- Vonderahe, A.R. Corneal and scleral anesthesia of the lower hale of the eye in a case of trauma of the superior maxillary nerve. Arch. Neurol. Psychiatry 1928, 20, 836–837.
- Ruskell, G.L. Ocular fibers of the maxillary nerve in monkeys. J. Anat. 1974, 118, 195–203.
- Marfurt, C.F.; Ellis, L.C. Immunohistochemical localization of tyrosine hydroxylase in corneal nerves. J. Comp. Neurol. 1993, 336, 517–531.
- Ehinger, B. Connections between Adrenergic Nerves and other Tissue Components in the Eye. Acta Physiol. Scand. 1966, 67, 57–64.
- Morgan, C.; DeGroat, W.C.; Jannetta, P.J. Sympathetic innervation of the cornea from the superior cervical ganglion. An HRP study in the cat. J. Auton. Nerv. Syst. 1987, 20, 179–183.
- Marfurt, C.F.; Kingsley, R.E.; Echtenkamp, S.E. Sensory and sympathetic innervation of the mammalian cornea. A retrograde tracing study. Investig. Ophthalmol. Vis. Sci. 1989, 30, 461–472.
- Toivanen, M.; Tervo, T.; Partanen, M.; Vannas, A.; Hervonen, A. Histochemical demonstration of adrenergic nerves in the stroma of human cornea. Investig. Ophthalmol. Vis. Sci. 1987, 28, 398–400.
- Marfurt, C.F.; Cox, J.; Deek, S.; Dvorscak, L. Anatomy of the human corneal innervation. Exp. Eye Res. 2010, 90, 478–492.
- He, J.; Bazan, N.G.; Bazan, H.E.P. Mapping the entire human corneal nerve architecture. Exp. Eye Res. 2010, 91, 513–523.
- Marfurt, C.F. Nervous control of the cornea. In Nervous Control of the Eye; Harwood Academic Publishers: Amsterdam, The Netherlands, 2000; p. 41.
- Chan-Ling, T. Sensitivity and neural organization of the cat cornea. Investig. Ophthalmol. Vis. Sci. 1989, 30, 1075–1082.
- Zander, E.; Weddell, G. Observations on the innervation of the cornea. J. Anat. 1951, 85, 68–99.
- Ivanusic, J.J.; Wood, R.J.; Brock, J.A. Sensory and sympathetic innervation of the mouse and guinea pig corneal epithelium. J. Comp. Neurol. 2013, 521, 877–893.
- Mckenna, C.C.; Lwigale, P.Y. Innervation of the mouse cornea during development. Investig. Ophthalmol. Vis. Sci. 2011, 52, 30–35.
- Wang, C.; Fu, T.; Xia, C.; Li, Z. Changes in mouse corneal epithelial innervation with age. Investig. Ophthalmol. Vis. Sci. 2012, 53, 5077–5084.
- Stepp, M.A.; Tadvalkar, G.; Hakh, R.; Pal-Ghosh, S. Corneal epithelial cells function as surrogate Schwann cells for their sensory nerves. Glia 2017, 65, 851–863.
- Rózsa, A.J.; Beuerman, R.W. Density and organization of free nerve endings in the corneal epithelium of the rabbit. Pain 1982, 14, 105–120.
- Schimmelpfennig, B. Nerve structures in human central corneal epithelium. Graefe’s Arch. Clin. Exp. Ophthalmol. 1982, 218, 14–20.
- Belmonte, C.; Garcia-Hirschfeld, J.; Gallar, J. Neurobiology of ocular pain. Progr. Ret. Eye Res. 1997, 16, 117–156.
- Dua, H.S.; Watson, N.J.; Mathur, R.M.; Forrester, J.V. Corneal epithelial cell migration in humans: ‘hurricane and blizzard keratopathy’. Eye 1993, 7, 53–58.
- Al-Aqaba, M.A.; Fares, U.; Suleman, H.; Lowe, J.; Dua, H.S. Architecture and distribution of human corneal nerves. Br. J. Ophthalmol. 2010, 94, 784–789.
- Dvorscak, L.; Marfurt, C.F. Age-related changes in rat corneal epithelial nerve density. Investig. Ophthalmol. Vis. Sci. 2008, 49, 910–916.
- Nagasaki, T.; Zhao, J. Centripetal Movement of Corneal Epithelial Cells in the Normal Adult Mouse. Investig. Ophthalmol. Vis. Sci. 2003, 44, 558–566.
- Patel, D.V.; McGhee, C.N.J. Mapping of the normal human corneal sub-basal nerve plexus by in vivo laser scanning confocal microscopy. Investig. Ophthalmol. Vis. Sci. 2005, 46, 4485–4488.
- Alamri, A.S.; Wood, R.J.; Ivanusic, J.J.; Brock, J.A. The neurochemistry and morphology of functionally identified corneal polymodal nociceptors and cold thermoreceptors. PLoS ONE 2018, 13, e0195108.
- Belmonte, C.; Acosta, M.C.; Gallar, J. Neural basis of sensation in intact and injured corneas. Exp. Eye Res. 2004, 78, 513–525.
- Acosta, M.C.; Belmonte, C.; Gallar, J. Sensory experiences in humans and single-unit activity in cats evoked by polymodal stimulation of the cornea. J. Physiol. 2001, 534, 511–525.
- Bron, R.; Wood, R.J.; Brock, J.A.; Ivanusic, J.J. Piezo2 expression in corneal afferent neurons. J. Comp. Neurol. 2014, 522, 2967–2979.
- Fernández-Trillo, J.; Florez-Paz, D.; Íñigo-Portugués, A.; González-González, O.; del Campo, A.G.; González, A.; Viana, F.; Belmonte, C.; Gomis, A. Piezo2 mediates low-threshold mechanically evoked pain in the cornea. J. Neurosci. 2020, 40, 8976–8993.
- Belmonte, C.; Giraldez, F. Responses of cat corneal sensory receptors to mechanical and thermal stimulation. J. Physiol. 1981, 321, 355–368.
- Belmonte, C.; Gallar, J.; Pozo, M.A.; Rebollo, I. Excitation by irritant chemical substances of sensory afferent units in the cat’s cornea. J. Physiol. 1991, 437, 709–725.
- Chen, X.; Gallar, J.; Belmonte, C; Reduction by antiinflammatory drugs of the response of corneal sensory nerve fibers to chemicalirritation.. Investig. Ophthalmol. Vis. Sci. 1997, 38, 1944–1953, .
- Gallar, J.; Pozo, M.A.; Tuckett, R.P.; Belmonte, C.; Response of sensory units with unmyelinated fibres to mechanical, thermal and chemical stimulation of the cat’s cornea.. J. Physiol. 1993, 468, 609–622, .
- Gallar, J.; Acosta, M.C.; Gutiérrez, A.R.; Belmonte, C.; Impulse activity in corneal sensory nerve fibers after photorefractive keratectomy.. Investig. Ophthalmol. Vis. Sci. 2007, 48, 4033–4037, .
- Bessou, P.; Perl, E.R.; Response of cutaneous sensory units with unmyelinated fibers to noxious stimuli. . J. Neurophysiol. 1969, 32, 1025–1043, .
- Meyer, R.A.; Ringkamp, M.; Campbell, J.N.; Raja, S.N. . Peripheral mechanisms of cutaneous nociception. n Wall & Melzack’s Textbook of Pain; Elsevier: Amsterdam, The Netherlands, 2006; pp. 3–34.
- Carr, R.W.; Pianova, S.; Fernandez, J.; Fallon, J.B.; Belmonte, C.; Brock, J.A.; Effects of heating and cooling on nerve terminal impulses recorded from cold-sensitive receptors in the guinea-pig cornea.. J. Gen. Physiol. 2003, 121, 427–439, .
- Parra, A.; Gonzalez-Gonzalez, O.; Gallar, J.; Belmonte, C.; Tear fluid hyperosmolality increases nerve impulse activity of cold thermoreceptor endings of the cornea.. Pain 2014, 155, 1481–1491, .
- Acosta, M.C.; Luna, C.; Quirce, S.; Belmonte, C.; Gallar, J.; Changes in sensory activity of ocular surface sensory nerves during allergic keratoconjunctivitis.. Pain 2013, 154, 2353–2362, .
- Acosta, M.C.; Peral, A.; Luna, C.; Pintor, J.; Belmonte, C.; Gallar, J.; Tear secretion induced by selective stimulation of corneal and conjunctival sensory nerve fibers.. Investig. Ophthalmol. Vis. Sci. 2004, 45, 2333–2336, .
- Parra, A.; Madrid, R.; Echevarria, D.; del Olmo, S.; Morenilla-Palao, C.; Acosta, M.C.; Gallar, J.; Dhaka, A.; Viana, F.; Belmonte, C.; et al. Ocular surface wetness is regulated by TRPM8-dependent cold thermoreceptors of the cornea. . Nat. Med. 2010, 16, 1396–1399, .
- Quallo, T.; Vastani, N.; Horridge, E.; Gentry, C.; Parra, A.; Moss, S.; Viana, F.; Belmonte, C.; Andersson, D.A.; Bevan, S.; et al. TRPM8 is a neuronal osmosensor that regulates eye blinking in mice. . Nat. Commun. 2015, 6, 7150, .
- Alcalde, I.; Íñigo-Portugués, A.; González-González, O.; Almaraz, L.; Artime, E.; Morenilla-Palao, C.; Gallar, J.; Viana, F.; Merayo-Lloves, J.; Belmonte, C.; et al. Morphological and functional changes in TRPM8-expressing corneal cold thermoreceptor neurons during aging and their impact on tearing in mice.. J. Comp. Neurol. 2018, 526, 1859–1874, .
- Gallar, J.; Acosta, M.C.; Moilanen, J.A.O.; Holopainen, J.M.; Belmonte, C.; Tervo, T.M.T.; Recovery of corneal sensitivity to mechanical and chemical stimulation after laser in situ keratomileusis. . J. Refract. Surg. 2004, 20, 229–235, .
- Luna, C.; Quirce, S.; Aracil-Marco, A.; Belmonte, C.; Gallar, J.; Acosta, M.C.; Unilateral Corneal Insult Also Alters Sensory Nerve Activity in the Contralateral Eye.. Front. Med. 2021, 8, 2079, .
- Acosta, M.C.; Luna, C.; Quirce, S.; Belmonte, C.; Gallar, J.; Corneal sensory nerve activity in an experimental model of UV keratitis. . Investig. Ophthalmol. Vis. Sci. 2014, 55, 3403–3412, .
- Kovács, I.; Luna, C.; Quirce, S.; Mizerska, K.; Callejo, G.; Riestra, A.; Fernández-Sánchez, L.; Meseguer, V.M.; Cuenca, N.; Merayo-Lloves, J.; et al. Abnormal activity of corneal cold thermoreceptors underlies the unpleasant sensations in dry eye disease. . Pain 2016, 157, 399–417, .
- Belmonte, C.; Pain, Dryness, and Itch Sensations in Eye Surface Disorders Are Defined by a Balance between Inflammation and Sensory Nerve Injury.. Cornea 2019, 38, S11–S24, .
- Luna, C.; Mizerska, K.; Quirce, S.; Belmonte, C.; Gallar, J.; del Carmen Acosta, M.; Meseguer, V.; Sodium Channel Blockers Modulate Abnormal Activity of Regenerating Nociceptive Corneal Nerves After Surgical Lesion. . Investig. Opthalmol. Vis. Sci. 2021, 62, 2, .
- Gonzalez, G.G.; De la Rubia, P.G.; Gallar, J.; Belmonte, C.; Reduction of capsaicin-induced ocular pain and neurogenic inflammation by calcium antagonists. Investig. Ophthalmol. Vis. Sci. 1993, 34, 3329–3335, .
- Vitar, R.M.L.; Barbariga, M.; Fonteyne, P.; Bignami, F.; Rama, P.; Ferrari, G.; Modulating ocular surface pain through neurokinin-1 receptor blockade.. Investig. Ophthalmol. Vis. Sci. 2021, 62, 26, .
- Murata, Y.; Masuko, S.; Peripheral and central distribution of TRPV1, substance P and CGRP of rat corneal neurons. . Brain Res. 2006, 1085, 87–94, .
- Yang, L.; Mehta, J.; Liu, Y.C.; Corneal neuromediator profiles following laser refractive surgery. . Neural Regen. Res. 2021, 16, 2177–2183, .
- Zhang, X.; Mak, S.; Li, L.; Parra, A.; Denlinger, B.; Belmonte, C.; McNaughton, P.A.; Direct inhibition of the cold-Activated TRPM8 ion channel by Gα q.. Nat. Cell Biol. 2012, 14, 850–858, .
