Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Physiology
Sodium retention is a well-documented consequence of many pathophysiological conditions, especially kidney disease, which is clinically recognized as an accumulation of edema.
- kidney
- lymphatics
- sodium
1. Proteinuric Kidney Injury Increased Renal Sodium
MRI analysis revealed that puromycin aminonucleoside nephropathy (PAN) injury leads to increased renal sodium content. Both the cortex and medulla in PAN-injured rats had significantly higher sodium than in control rats (Figure 1A). The renal cortex of PAN rats also showed increased water content compared with controls. Although a directionally similar trend occurred in the medulla of PAN-injured rats, the increase did not reach statistical significance (Figure 1B). In companion studies, we measured the sodium concentration in the lymph exiting the kidney, which is thought to reflect the composition within the renal interstitial compartment. These direct measurements revealed that sodium concentration in the renal lymph of PAN rats was significantly higher than in the lymph of control rats (Figure 2A). Sodium concentration in concurrently obtained serum samples was not different between PAN rats and controls (Figure 2B). These results indicate that, in addition to the well-documented proteinuria, hypoalbuminemia, and hyperlipidemia, PAN kidney injury leads to intrarenal sodium and water retention, especially in the renal cortex. Similar to dermal lymphatics of hypertensive animals, which transport excess sodium from the skin [1], our results show for the first time that renal lymphatics are a route for clearing excess sodium from the renal interstitium in the setting of proteinuric kidney injury.
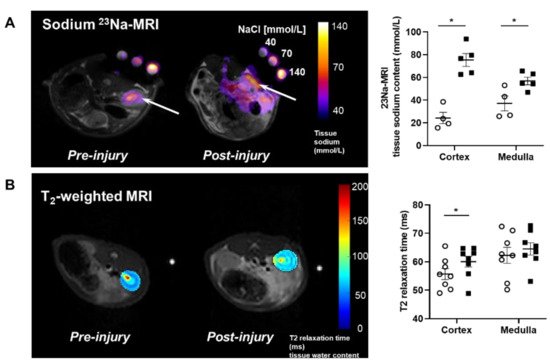
Figure 1. Proteinuric kidney injury increased renal sodium and water content. (A) Representative sodium 23Na-MRI in uninjured control (Cont) (open circles) and puromycin (PAN)-injured rats (closed squares). The graph shows mean tissue sodium content localized in the cortex and medulla of the kidneys (arrows). (B) Representative T2-weighted MRI images and quantitative T2-relaxation time measurements indicative of renal cortical and medullary water content in Cont and PAN-injured rats. Results are expressed as mean ± SEM. n = 5 to 8 rats per group analyzed by unpaired t test. * p < 0.05.
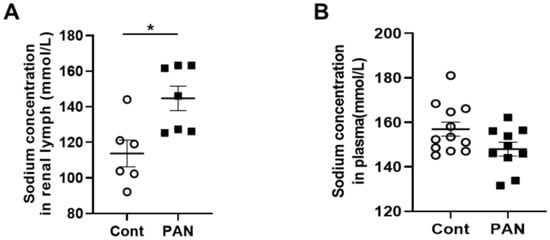
Figure 2. Proteinuric kidney injury increased sodium concentration in renal lymph. (A) Analysis of renal lymph showed higher sodium concentration in PAN-injured (closed squares) vs control rats (open circles). (B) Analysis of plasma showed no difference in sodium concentration between PAN-injured vs control rats. Results are expressed as mean ± SEM for 6 to 8 rats per group analyzed by unpaired t test. * p < 0.05.
2. High Sodium Environment Changed Contractility of Renal Lymphatic Vessels Involving the NKCC1 Transporter
To determine the effects of a high-sodium environment on renal lymphatic function, we measured the vasodynamics of renal afferent collecting lymphatic vessels. Similar to studies in afferent skin lymphatics [2], the contractility was assessed in renal afferent vessels isolated from normal rats exposed to normal buffer containing 143 mmol Na+ Krebs solution and compared with dynamics following exposure to Krebs solution containing a sodium concentration of 185 mmol. Compared with the physiologic buffer, a high-sodium environment had little effect on lymphatic contraction frequency. However, although high sodium caused only subtle changes in end diastolic diameter (EDD), a pronounced increase in end systolic diameter (ESD) contributed to reduced contraction amplitude and ejection fraction compared with the physiologic buffer (Figure 3).
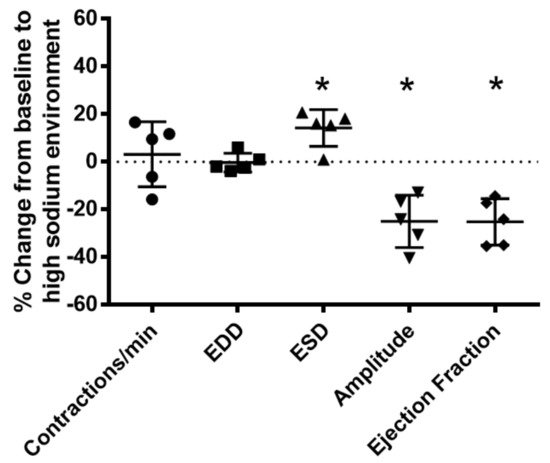
Figure 3. High salt environment altered renal collecting lymphatic vessel pumping function. Extra−renal afferent lymphatic vessels were subjected to a high−sodium buffer (185 mmol Na+ Krebs solution). A digital image capture system was used to measure the following vessel pumping parameters: frequency of spontaneous contractions, end diastolic lumen diameter (EDD), end systolic lumen diameter (ESD), contraction amplitude, and ejection fraction. Exposure to a high−sodium environment caused a significant increase in ESD, resulting in a significant decrease in amplitude and ejection fraction. Data points represent the percent change from measurements captured under baseline conditions (143 mmol Na+ Krebs solution) and are expressed as mean ± SEM. n = 5 individual vessels isolated from 5 rats. Significance was assessed by analyzing raw measurements using an unpaired t test. * p < 0.05.
NKCC1 regulates blood vessel dynamics, and our previous study confirmed expression of NKCC1 in lymphatic endothelial cells. However, it is unknown whether NKCC1 has a role in modulating microenvironmental influences on lymphatic vessel dynamics. This is interesting, as lymphatic vessels were recently reported to regulate sodium homeostasis. Renal lymphangiogenesis in mice with kidney-specific overexpression of VEGF-D increased urinary sodium excretion and reduced systemic blood pressure in salt-loaded hypertensive mice but not normotensive basal conditions [3]. The mechanism was linked to downregulation of sodium transporters, namely, total NCC and ENaCα in tubular epithelial cells. NKCC1 expression and renal lymphatic function were not evaluated. Our immunohistochemical staining verified prominent expression of NKCC1 in afferent renal lymphatic vessels (Figure 4A). Moreover, NKCC1 gene expression was increased in vessels from PAN-injured rats compared with controls (Figure 4B). Also, LECs exposed to a high-sodium environment had elevated NKCC1 mRNA compared with cells maintained in media with physiological levels of sodium (Figure 5A). Similar upregulation in NCKK1 mRNA occurred in response to urea that is equimolar to high sodium exposure. Since NKCC activity is determined by phosphorylation, we assessed phosphorated-NKCC1 protein. Our results show that a high-sodium environment significantly reduced expression of phosphorated-NKCC1 protein while the hyperosmolar urea control did not (Figure 5A). Furthermore, as NKCC activity is linked to phosphorylation of WNK-SPAK/OSR1 signaling cascade, we also examined expression of this upstream kinase [4][5]. Our data show that a high-sodium environment also reduced phosphorylated SPAK compared with the baseline sodium control group (Figure 5C) [4]. Among the vasoactive factors, eNOS is a major endothelial-derived mechanism regulating lymphatic dynamics, which is regulated by activity of NKCC1 [6]. Exposing LECs to a high-sodium environment caused a significant reduction in eNOS activity as measured by the amount of phosphorylated eNOS protein (Figure 6A). In contrast, increased osmolarity with urea did not significantly alter the endothelial eNOS activity although the eNOS activity was significantly higher than in cells exposed to a high-sodium environment, echoing reduced p-eNOS levels shown in cardiac tissues of rats fed a high-salt diet [7]. To determine the consequences of reduced NO signaling on renal lymphatic vessel pumping dynamics, we exposed isolated vessels to L-NAME in order to inhibit eNOS activity. This caused a significant increase in contraction frequency, but reduced EDD, magnitude of contraction, and ejection fraction (Figure 6B).
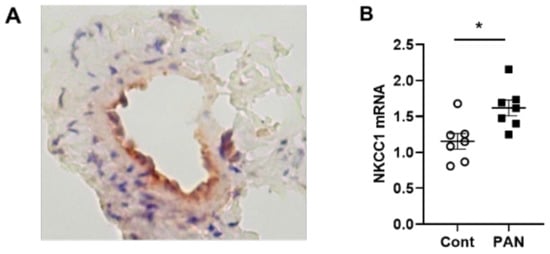
Figure 4. NKCC-1 transporter expression in renal lymphatic vessels and vessels with PAN proteinuric injury. (A) Immunostaining of afferent renal lymphatic vessels demonstrated NKCC1 transporter expression, particularly prominent in lymphatic endothelial cells. (B) NKCC1 mRNA levels in extra-renal lymphatic vessels were significantly greater in PAN-injured rats vs uninjured controls. Results are mean ± SEM for 7 rats per group analyzed by unpaired t test * p < 0.05.
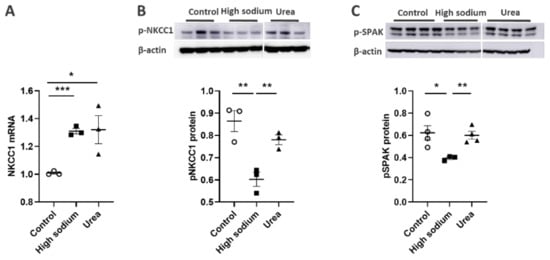
Figure 5. High Na+ environment regulated NKCC-1 signaling pathway in lymphatic endothelial cells (LECs). (A) Cultured LECs exposed to a high-sodium environment showed greater expression of NKCC1 mRNA, (B) while expression of phosphorated NKCC-1 protein decreased. (C) High-sodium environment decreased protein expression of SPAK, an upstream activating kinase of NKCC1. Experiments were performed independently 3 times using 3 wells per treatment and analyzed by ANOVA followed by Dunnett multiple comparisons. * p < 0.05, ** p < 0.01, *** p < 0.001.
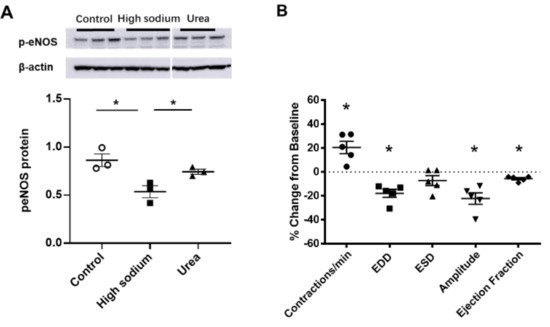
Figure 6. eNOS modulated lymphatic vessel function. (A) Cultured LECs exposed to high-sodium, but not high-osmolar environment showed reduced eNOS activity. (B) Isolated renal lymphatic vessels challenged with the eNOS inhibitor, L-NAME, exhibited increased contraction frequency and reduced EDD, amplitude, and ejection fraction. EDD, end diastolic diameter; ESD, end systolic diameter. Protein concentration results are expressed as mean ± SEM for 3 samples analyzed by ANOVA followed by Dunnett multiple comparison test. Vessel pumping parameters are expressed as the percent change from measurements captured under baseline conditions (143 mmol Na+ Krebs solution) and are expressed as mean ± SEM for 5 individual vessels isolated from 5 rats. Significance was assessed by analyzing raw measurements using an unpaired t test. * p < 0.05.
3. Kidney Injury Diminished the Lymphatic Vascular Response to a High-Sodium Environment and NKCC Inhibition by Furosemide
To gain further insight into the effects of kidney injury on renal lymphatic physiology, we compared the pumping dynamics of control vessels with vessels from a PAN-injured rat in a normal sodium environment (Figure 7). PAN-injured vessels had a significant increase in EDD (Figure 7B), which contributed to a marked decrease in contraction amplitude (Figure 7D) and ejection fraction (Figure 7E) compared with control vessels. Next, to investigate how a high-sodium environment affects vessels in the setting of kidney injury, we compared the lymphatic dynamics in the vessels of PAN-injured rats before and after exposure to a high-sodium environment (Figure 8). PAN-injured lymphatic vessels had a distinct response to a high-sodium environment compared with the response of control vessels (Figure 3), exhibiting a decreased EDD, and a decreased ejection fraction. This suggests that a high-sodium environment and PAN injury both result in reduced ejection fraction, albeit by different mechanisms.
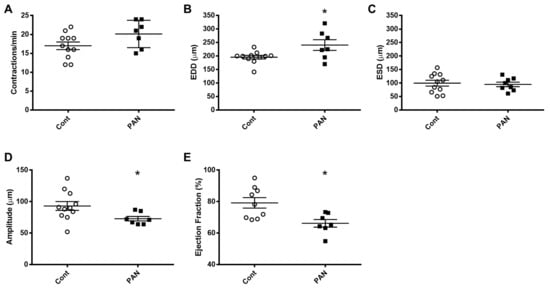
Figure 7. Kidney injury diminishes lymphatic vessel pumping efficiency. Vasodynamic parameters were measured in renal lymphatic vessels isolated from control and PAN-injured rats. Vessels from PAN-injured rats had significantly increased EDD (B), resulting in reduced contraction amplitude (D) and ejection fraction (E), while contraction frequency (A) and ESD (C) remained unchanged. Datapoints represent raw measurements from individual vessels isolated from 7 to 11 rats per group. Results are expressed as mean ± SEM analyzed by unpaired t test. * p < 0.05 EDD, end diastolic diameter; ESD, end systolic diameter.
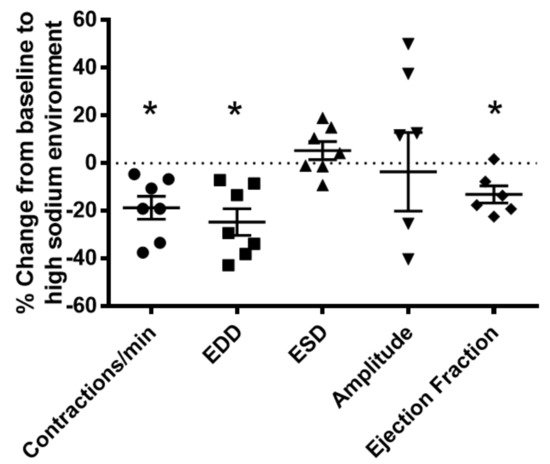
Figure 8. Vasodynamic response of PAN-injured lymphatic vessels exposed to a high-sodium environment. PAN-injured vessels exposed to high sodium had a significant decrease in the frequency of spontaneous contractions, EDD, and ejection compared with PAN-injured vessels in a normal sodium environment. Data points represent the percent change from measurements captured under normal sodium conditions and are expressed as mean ± SEM for 6 to 7 individual vessels isolated from 6 to 7 rats. Significance was assessed by analyzing raw measurements using an unpaired t test. * p < 0.05. EDD, end diastolic diameter; ESD, end systolic diameter.
Since injured lymphatic vessels appear to have weaker intrinsic compensatory responses to the high-sodium environment likely prevailing in a disease setting, we examined the effects of the NKCC1 inhibitor furosemide in PAN-injured and control vessels. Control vessels treated with furosemide had a pronounced concentration-dependent decrease in ejection fraction. In contrast, furosemide had more subtle effects on the ejection fraction of PAN-injured vessels, with PAN vessels being significantly less affected by furosemide at physiologically relevant doses (Figure 9). Interestingly, there was no statistical difference in the effects of furosemide on PAN vessels in a normal or high-sodium environment. These results indicate that analogous to a high-sodium environment and PAN-induced kidney injury, furosemide exerts directionally similar moderating effects on lymphatic dynamics that may affect renal interstitial clearance.
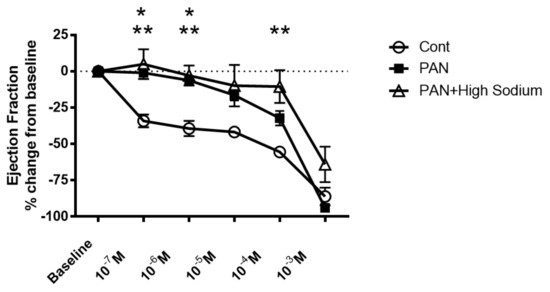
Figure 9. Kidney injury and exposure to elevated sodium blunt lymphatic vessel response to NKCC inhibition by furosemide. Renal lymphatic vessels isolated from PAN-injured rats and normal controls were subjected to increasing concentrations of the NKCC antagonist furosemide. Some PAN vessels were challenged with furosemide in a high-sodium environment. In control vessels, furosemide induces a robust decrease in ejection fraction. In contrast, PAN-injured vessels in normal and high-sodium environments are significantly less sensitive to furosemide. Data points represent the percent change from measurements captured at baseline conditions and are expressed as mean ± SEM for <6 individual vessels isolated from <6 rats. Significance (p < 0.05) was analyzed by ANOVA followed by Dunnett multiple comparisons. * PAN vessels compared with control vessels, ** PAN vessels in high sodium compared with control vessels.
This entry is adapted from the peer-reviewed paper 10.3390/ijms23031428
References
- Wiig, H.; Schröder, A.; Neuhofer, W.; Jantsch, J.; Kopp, C.; Karlsen, T.V.; Boschmann, M.; Goss, J.; Bry, M.; Rakova, N.; et al. Immune Cells Control Skin Lymphatic Electrolyte Homeostasis and Blood Pressure. J. Clin. Investig. 2013, 123, 2803–2815.
- Karlsen, T.V.; Nikpey, E.; Han, J.; Reikvam, T.; Rakova, N.; Castorena-Gonzalez, J.A.; Davis, M.J.; Titze, J.M.; Tenstad, O.; Wiig, H. High-Salt Diet Causes Expansion of the Lymphatic Network and Increased Lymph Flow in Skin and Muscle of Rats. Arter. Thromb. Vasc. Biol. 2018, 38, 2054–2064.
- Balasubbramanian, D.; Baranwal, G.; Clark, M.C.; Goodlett, B.L.; Mitchell, B.M.; Rutkowski, J.M. Kidney-specific lymphangiogenesis increases sodium excretion and lowers blood pressure in mice. J. Hypertens. 2020, 38, 874–885.
- Delpire, E.; Gagnon, K.B. Na(+)-K(+)-2cl(−) Cotransporter (Nkcc) Physiological Function in Nonpolarized Cells and Transporting Epithelia. Compr. Physiol. 2018, 8, 871–901.
- Zeniya, M.; Sohara, E.; Kita, S.; Iwamoto, T.; Susa, K.; Mori, T.; Oi, K.; Chiga, M.; Takahashi, D.; Yang, S.S.; et al. Dietary Salt Intake Regulates WNK3-SPAK-NKCC1 Phosphorylation Cascade in Mouse Aorta through Angiotensin II. Hypertension 2013, 62, 872–878.
- Baldwin, S.N.; Sandow, S.L.; Mondéjar-Parreño, G.; Stott, J.B.; Greenwood, I.A. K(V)7 Channel Expression and Function within Rat Mesenteric Endothelial Cells. Front. Physiol. 2020, 11, 598779.
- Li, Y.; Wu, X.; Mao, Y.; Liu, C.; Wu, Y.; Tang, J.; Zhao, K.; Li, P. Nitric Oxide Alleviated High Salt-Induced Cardiomyocyte Apoptosis and Autophagy Independent of Blood Pressure in Rats. Front. Cell Dev. Biol. 2021, 9, 646575.
This entry is offline, you can click here to edit this entry!
